Abstract
Background: To avoid aggressive treatments at the end-of-life and to provide palliative care (PC), physicians need to terminate futile anti-cancer treatments and define the palliative goal of the treatment in time. This single center study assesses the practices used to make the decision that leads to treatment with a palliative goal, i.e., the PC decision and its effect on anti-cancer treatments at the end of life.
Material and methods: Patients with a cancer diagnosis treated in tertiary hospital during 1st January 2013 – 31st December 2014 and deceased by the end of 2014 were identified in the hospital database (N = 2737). Of these patients, 992 were randomly selected for this study. The PC decision was screened from patient records, i.e., termination of cancer-specific treatments and a focus on symptom-centered PC.
Results: The PC decision was defined in 82% of the patients during the last year of life (49% >30 days and 33% ≤30 days before death, 18% with no decision). The median time from the decision to death was 46 days. Systemic cancer therapy was given during the last month of life in 1%, 36% and 38% (p < .001) and radiotherapy 22%, 40% and 31% (p = .03) cases, respectively; referral to a PC unit was made in 62%, 22% and 11%, respectively (p < .001). In logistic regression analyses younger age, shorter duration of the disease trajectory and type of cancer (e.g., breast cancer) were associated with a lack or late timing of the PC decision.
Conclusion: The decision to initiate a palliative goal for the treatment was frequently made for cancer patients but occurred late for every third patient. Younger age and certain cancer types were associated with late PC decisions, thus leading to anti-cancer treatments continuing until close to the death with low access to a PC unit.
Background
Anti-cancer treatments may have a detrimental effect on the quality of life of fragile cancer patients. Aggressive treatments during end-of-life (EOL) also impose considerable costs on health care systems [Citation1]. Even though treatment practices may differ between countries, studies from the US and Canada suggest that the trend for aggressive EOL cancer care is increasing [Citation2,Citation3]. Proposed standard benchmarks for ‘not overly aggressive cancer care’ include less than 10% of patients receiving chemotherapy 14 days prior to death and less than 2% of patients starting a new chemotherapy regimen 30 days prior to death [Citation4]. The response to preceding with anti-cancer treatments in advanced stages of cancer is often uncertain whereas the toxicity increases the decline in performance status. In order to avoid futile treatments at the EOL, the treatment focus should be on symptom-centered PC rather than on life-prolongation.
There is no clear agreement on the indicators of the overuse of radiotherapy during the final weeks of life because of its palliative nature in some cases. However, it has been suggested that radiotherapy administered during the last 14 or 30 days of life might be a useful quality indicator [Citation5] as there is often insufficient time to reach a response.
The utilization of EOL chemotherapy and radiotherapy has previously been investigated [Citation6–22], but there are no studies on the impact of PC decision-making and timing on anti-cancer treatments at the EOL.
In the present study, we determined the prevalence and timing of PC decision in relation to the use of anti-cancer treatments during the last year of life. The secondary aim was to investigate which factors affected the decision-making.
Material and methods
Cohort selection
Patients with a cancer diagnosis (ICD-10 C00-C96) treated in the Department of Oncology during 1 January .2013–31 December 2013 and deceased by 31 December 2014 were identified (N = 2737) from the Helsinki University Central Hospital (HUCH) database. Of these patients, 992 were randomly selected for the study cohort. The final study sample consisted of 949 patients, after 43 patients were excluded because their primary cause of death was other than cancer or they were pediatric (<18-year old) cancer patients.
This historic registry-based study was done with the permission of the authorities of HUCH. According to the Finnish registration for research, no ethics committee approval was needed.
The majority of Finnish cancer patients are initially evaluated at public university and central hospitals. HUCH is one of the five university hospitals in Finland and provides cancer care for approximately 1.6 million residents in Southern Finland. During the time of this study, the HUCH Department of Oncology was responsible for the radiation therapy treatments of all cancer patients and the systemic cancer treatments of all except pediatric, hematological, gynecological and lung cancer patients; these patients received systemic therapy in other departments. In the Department of Oncology, there is a PC outpatient unit, but municipalities are responsible for EOL care. Early integrated PC was not systematically organized at the time of the study.
Data sources and collection
The cohort of patients and their respective clinical information were identified from electronic medical records. The patient-level data included age, gender, and cancer diagnosis, oncological systemic treatments, radiation therapy, visits to the PC unit, PC decision (date) and date of death.
Patients were divided into 13 diagnosis groups: (i) head and neck cancers, (ii) upper gastro-intestinal (GI) cancers, (iii) colorectal cancers, (iv) lung cancer, (v) melanoma and other skin cancers, (vi) breast cancer, (vii) gynecological cancers, (viii) prostate cancer, (ix) sarcomas, (x) cancers of the urinary tract, (xi) primary CNS malignancies, (xii) lymphomas and (xiii) others. When a deceased patient had more than one malignancy, the cancer diagnosis was recorded in accordance with the primary cause of death. The primary cause of death was collected from the death certificates of the national death certificate registry of Statistics Finland.
Most data used in this study was available in a structured format, but some information was manually derived from the medical records, e.g., the PC decision. The two researchers reviewed all medical records according to the study protocol. The consistency of the derived recordings was randomly cross-checked. One researcher is a PhD and a specialist in medical oncology and the other is a PhD student and a general practitioner. Due to the nature of the data, there were no missing values: diagnoses, dates and treatments given to the patients are mandatory information to be recorded.
Palliative care decision and period
The PC decision, i.e., the decision to terminate life prolonging anti-cancer treatments and focus on symptom centered PC, is made by the oncologist responsible for the care of the patient. The PC period was initiated by the PC decision and defined as the period of time after termination of cancer-specific chemotherapy or biological treatments. However, endocrine treatment could continue during the palliative period, e.g., LHRH analog for prostate cancer patients. In addition, short courses of palliative radiotherapy, e.g., to relieve pain were allowed. A search for the PC decision was made in the medical records of all patients. The decision was recorded if there was an explicit and clear mention of it in medical records.
Patients were divided into three groups based on the timing of the PC decision: (1) no explicit PC decision, (2) the PC decision made during the last 30 days before death and (3) the PC decision made more than 30 days before death. The variables studied for each segment were: a visit to the palliative care (PC) unit and the last systemic cancer treatment and radiation therapy given before death. The date of the last radiation therapy was defined as the date of the last fraction and for systemic cancer treatment the last date of the treatment administered in hospital. With orally administered systemic cancer treatment, the last day of treatment was defined as the date when the oncologist recorded the termination of the treatment in the medical records.
Statistical analysis
Descriptive statistics such as means and medians were used for patient characteristics. The influence of the PC decision on treatments was studied by testing the difference between the three groups with cross-tabulation. The statistical significance of the differences of the distributions was tested with a chi-squared test. A one-way ANOVA was used to analyze whether the age groups significantly differed depending on the duration of time between the PC decision and death. ANOVA was also used to analyze pairwise whether the three groups of patients (based on timing of the PC decision) differed with respect to receiving radiation therapy before death. The same analysis of differences in systemic cancer treatments before death was done using the Kruskall-Wallis test because the variances in the groups were not equal. A logarithmic transformation was performed to normalize the distribution of time between the PC decision and death. A logarithmic regression model was built to show the factors associated to the PC decision. The dependent variable was defined as whether the PC decision was made earlier than 30 days prior to death or not. The variables tested in the model were age, cancer diagnosis and time from diagnosis to death. Both age and time from diagnosis to death were standardized (average = 0, stdev =1) to normalize the distributions. Gender was not included due to its perfect correlation with gender specific cancer diagnoses. Two-sided p values less than .05 were accepted as statistically significant. All analyses were conducted with SPSS (version 25).
Results
There were 949 patients in the final sample, out of which 53% were male (). The key characteristics of the patient population are presented in .
Table 1. Characteristics of the patient population.
Decision for PC was made >30 d and ≤30 d before death in 49% and 33% of the patients, respectively. No PC decision was made for 18% of the patients.
The distribution of patients in the three groups varied depending on the cancer diagnosis (). Most often the PC decisions were made earlier than 30 days prior to death for patients with colorectal cancer, sarcomas and prostate cancer, while the decisions were typically made within the last month before death for breast and skin cancer patients. For 61% of head and neck cancer patients, a PC decision was made at any point in time. The differences in the distributions between cancers were statistically significant (p = .02).
Table 2. Time from palliative care decision to death in different cancer groups.
The distribution of patients into the three groups depending on the patient’s age at death is presented in . There were statistically significant differences between the age groups (F-value 5,186, p value < .001), but only the group of patients >80-year old were statistically different from other age groups (p < .01 for all pairwise comparisons).
Table 3. Time from PC decision to death in different age groups.
Characteristics associated with a PC decision made >30 days before death in a logarithmic regression model are shown in . As the proportion of patients receiving a PC decision >30 d before death was lowest in breast cancer patients, we selected the breast cancer as the reference category for cancer groups. A statistically significant positive association was found for age (OR 1.3) and number of days from diagnosis to death (OR 1.2). Thus, the PC decision was more probable >30 days before death the older the patient and the longer the disease trajectory. From the diagnoses, the odds were highest for colorectal cancers and sarcomas (OR 5.2 and OR 4.3, respectively), hence these diagnoses were associated most often with the PC decision >30 days before death.
Table 4. Characteristics associated with a PC decision made > 30 days before death in the logistic regression model: odds ratios and confidence intervals.
illustrates the association between the PC decision and its timing to the last radiation therapy visit or last course of systemic cancer treatment before death. The analysis was done only for the patients that received radiation therapy (n = 468) or systemic cancer treatments (n = 580) during the last year of life. Of these patients, 29% and 20% received radiation therapy and systemic cancer treatment during the last month of life, respectively. However, for those receiving radiation therapy, the goal of the last treatment was palliative for 87%. The majority of the patients who received radiation therapy or systemic cancer treatment during the last month did not have a PC decision or the decision was made within the last month. Comparing the distributions in , the chi-squared test only indicated a statistically significant difference in the distributions for the systemic cancer treatments (p < .001 for systemic cancer treatments and p = .03 for radiation therapy). Pairwise comparisons revealed that for radiation therapy the difference between the three patient groups was statistically significant only between those with a PC decision made over 30 days prior to death and those with a PC decision within one month of death (p = .003). For systemic cancer treatment, the differences in the distributions were statistically significant between those with a PC decision made over 30 days prior to death and both other groups (p < .001). The difference between the two other patient groups, however, was not statistically significant (data not shown).
Figure 1. Time between last radiation therapy and death (a) and time between last systemic cancer treatment and death (b) with respect to the timing of the PC decision.
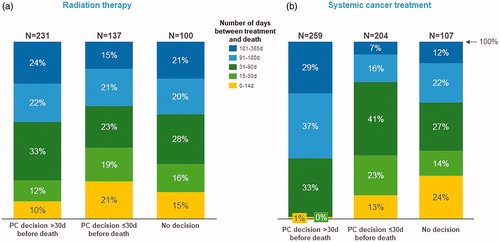
Sixty-two percent of the patients with a PC decision made >30 days before death visited the PC unit as compared to 22% of the patients with the decision ≤30 days and 11% without the decision. The differences between the groups were statistically significant (p < .001).
Discussion
In this assessment of EOL care at a Finnish university hospital, a PC decision defining the symptom-centered goal of the care was frequently made, but often occurred late in the disease trajectory. Patients with no or a very late decision received more aggressive cancer treatments at the EOL and made infrequent visits to the PC unit. Age, duration of the disease trajectory and type of cancer were associated with the timing of the decision.
Frequency and timing of PC decision
According to previous studies, only a minority of patients with a prognosis of less than one year, have discussed EOL care with their oncologist [Citation23]. In the present study, a PC decision was found in the medical records of 82% of the patients, which is a relatively high figure. However, even though discussions about the termination of anti-cancer treatments were carried out prior to death in a majority of the patients, the decision was only made at least a month before death for half of the patients.
A PC decision was more likely to be made for older patients with the threshold being patients over 80 years of age. In a previous Finnish study [Citation16], patients under the age of 50 were more likely to receive IV chemotherapy during the last months and weeks of life compared to older patients. Likewise, Braga et al. [Citation7] have shown the increased probability of younger patients receiving chemotherapy during the last three months of life. Although our results are in line with these earlier studies, the difference was significant only in the very elderly population (above 80 years) in our study.
As well as the younger age of the patient, the chemosensitivity of the tumor and thus the available alternative treatment options have been found to correlate with the use of chemotherapy during the last months of life [Citation3,Citation7,Citation8,Citation12]. Similarly, in the present study, among the breast cancer patients the decision to terminate cancer-specific treatment, i.e., making a PC decision was done late. On the other hand, the short disease trajectory, like in head and neck cancer, decreases the likelihood of early PC decision [Citation24–26]. Thus, in diseases with a very poor prognosis the integration of PC should take place early, i.e., immediately after the diagnosis of an advanced disease. For diseases with multiple treatment options and a relatively long survival, PC should be introduced at least when the treatment options diminish [Citation27].
Impact of PC decision on cancer treatments in EOL
Chemotherapy late in life does not improve patient’s quality of life [Citation28], in fact it is quite the contrary; it has been shown that a patient receiving palliative chemotherapy will most likely require more admissions to hospital, have a lower chance of dying at home, and may also have a shortened survival time [Citation9].
In this study, 20% of the patients received systemic cancer treatment during the last month of life. This was in line with another Finnish study [Citation16] where 18% of the patients received IV chemotherapy during the last month. The corresponding figures during the last two weeks of life were 18% in this study and 7% in the study by Rautakorpi et al. [Citation16]. However, in the present study orally given chemotherapy and biological treatments were also included, which could explain the higher rate of patients receiving systemic treatment at the EOL. In previous studies, the prevalence of anti-cancer treatments one month before death have varied from 12% to 43% [Citation2,Citation7,Citation9–15]. Comparison between the studies is, however, challenging, as the health care systems, study designs, and inclusion criteria vary. Nevertheless, according to proposed standard benchmarks for ‘not overly aggressive cancer care’, no more than 10% of patients should receive chemotherapy 14 days prior to death [Citation4]. Our results showed that the timing of the PC decision has a distinct correlation with the use of systemic anti-cancer treatments at EOL. If the PC decision was left undone or postponed to the last month of life the risk of aggressive cancer treatments during the EOL was significantly higher compared to patients with an early PC (38%, 36% and 1%, respectively). Interestingly, the risk of aggressive treatment at EOL was as high with a late PC decision as without it.
Thus, not only making the decision to switch to care with a symptom-centered palliative goal is important but its timing is also of vital importance. An early decision enables not only timely advance care planning and access to PC, but also the termination of futile, and potentially quality of life reducing systemic anti-cancer treatments.
In the present study, 29% of the patients received radiotherapy during the last month of life and 14% in the last two weeks, respectively. The prevalence of radiotherapy in the present study is somewhat higher than in the previous studies. In another Finnish study from 2005 to 2013 [Citation22], the corresponding figures were 23% and 12%, respectively. According to previous studies, the proportion of patients receiving radiotherapy one month prior to death varied between 5% and 12% [Citation6,Citation15,Citation17–19]. Interestingly, there was no difference in the number of radiotherapy treatments at the EOL with regards to the timing of the PC decision. One explanation for higher portion of patients getting radiotherapy at the EOL in this study could be the high proportion (87%) of palliative radiotherapy in the present population. However, according to the study by Rautakorpi et al. nearly half of the treatments given in the last two weeks were discontinued, the deterioration of the general condition being the most common reason for the discontinuation [Citation22]. In addition, if radiotherapy is given very late, patients do not obtain any benefit from the treatment [Citation29,Citation30].
Impact of PC decisions on visits in PC unit
Even though we did not study the quality of EOL care, referral to a PC unit could be considered as an indicator of better EOL care [Citation31–33]. In the present study, the earlier the decision of a palliative goal for the treatment was made the more often the patient was referred to a PC unit (62% with early, 22% with late and 11% with no PC decision). In HUCH, the PC decision is made by the oncologist responsible for the cancer treatments instead of a shared decision making with the palliative team as during the study period no systematic early-integrated PC was offered in the center. According to an international consensus, one of the indicators required for decision making is referral to a palliative team [Citation27]. Our findings emphasize the importance of early integration of PC to improve EOL care planning [Citation31–34].
Limitations and strengths of the study
There are some limitations to our study. One limitation is the retrospective nature of our study; the data was based on hospital medical records. Any possible inaccuracies in the records might also be reflected in the results. However, the information concerning the PC decision and the time it was made was searched for manually and thus reliable. The other limitation is the lack of data on the response to last anti-cancer therapies. Neither did we search for the treatment intention (curative or disease modifying) in systemic cancer therapies at the EOL in relation to PC decision and its timing. Due to the retrospective nature of the study, the data on the quality of life and the need for palliative interventions is missing. Furthermore, we were unable to control any variables outside medical records, such as patient’s socioeconomic status or ethnic group. The strength of the study is that it is a population-based real-life study with a relatively large sample size. The study cohort was epidemiologically representative of the prominent oncological diseases found within the population. The information concerning the PC decision made by the oncologists and its timing in relation to death, in addition to the data on the referral to a PC unit provided unique information about the decision making at the end of life.
Conclusions
Our study revealed that for most cancer patients the PC decision as regards a symptom-centered palliative goal for their care was made by the oncologists. However, the decision was made very late. The lack of a PC decision or postponing it to the last month of life reflected a significantly increased risk for aggressive cancer treatments during the EOL and delayed the access to PC services. Early-integrated PC should be offered more systematically to ensure timely advanced care planning and access to palliative and EOL care.
Acknowledgments
Elizabeth Nyman is acknowledged for language revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576.
- Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321.
- Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–1591.
- Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17:505–509.
- Jones JA, Lutz ST, Chow E, et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296–310.
- Asola R, Huhtala H, Holli K. Intensity of diagnostic and treatment activities during the end of life of patients with advanced breast cancer. Breast Cancer Res Treat. 2006;100:77–82.
- Braga S, Miranda A, Fonseca R, et al. The aggressiveness of cancer care in the last three months of life: a retrospective single centre analysis. Psychooncology. 2007;16:863–868.
- Kao S, Shafiq J, Vardy J, et al. Use of chemotherapy at end of life in oncology patients. Ann Oncol. 2009;20:1555–1559.
- Näppa U, Lindqvist O, Rasmussen BH, et al. Palliative chemotherapy during the last month of life. Ann Oncol. 2011;22:2375–2380.
- Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394–400.
- Numico G, Trogu A, Cristofano A, et al. Active treatment given in the last weeks of life: poor quality cancer care or justifiable behavior?. Support Care Cancer. 2014;22:2813–2819.
- Adam H, Hug S, Bosshard G. Chemotherapy near the end of life: a retrospective single-centre analysis of patients' charts. BMC Palliat Care. 2014;13:26.
- Lee HS, Chun KH, Moon D, et al. Trends in receiving chemotherapy for advanced cancer patients at the end of life. BMC Palliat Care. 2015;14:4.
- Pacetti P, Paganini G, Orlandi M, et al. Chemotherapy in the last 30 days of life of advanced cancer patients. Support Care Cancer. 2015;23:3277–3280.
- Anshushaug M, Gynnild MA, Kaasa S, et al. Characterization of patients receiving palliative chemo- and radiotherapy during end of life at a regional cancer center in Norway. Acta Oncol. 2015;54:395–402.
- Rautakorpi LK, Seyednasrollah F, Mäkelä JM, et al. End-of-life chemotherapy use at a Finnish university hospital: a retrospective cohort study. Acta Oncol. 2017;56:1272–1276.
- Matter-Walstra KW, Achermann R, Rapold R, et al. Cancer-related therapies at the end of life in hospitalized cancer patients from four Swiss cantons: SAKK 89/09. Oncology. 2015;88:18–27.
- Guadagnolo BA, Liao KP, Elting L, et al. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31:80–87.
- Huang J, Wai ES, Lau F, et al. Palliative radiotherapy utilization for cancer patients at end of life in British Columbia: retrospective cohort study. BMC Palliat Care. 2014;13:49.
- Murphy JD, Nelson LM, Chang DT, et al. Patterns of care in palliative radiotherapy: a population-based study. J Oncol Pract. 2013;9:e220–e227.
- Lavergne MR, Johnston GM, Gao J, et al. Variation in the use of palliative radiotherapy at end of life: examining demographic, clinical, health service, and geographic factors in a population-based study. Palliat Med. 2011;25:101–110.
- Rautakorpi LK, Mäkelä JM, Seyednasrollah F, et al. Assessing the utilization of radiotherapy near end of life at a Finnish University Hospital: a restrospective cohort study. Acta Oncol. 2017;56:1265–1271.
- Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31:3315–3319.
- Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer. 2000;36:1032–1037.
- Ledeboer QC, van der Schroeff MP, Pruyn JF, et al. Survival of patients with palliative head and neck cancer. Head Neck. 2011;33:1021–1026.
- Heinonen T, Loimu V, Saarilahti K, et al. End-of-life pathway of head and neck cancer patients: single-institution experience. Eur Arch Otorhinolaryngol. 2018;275:545–551.
- Hui D, Mori M, Watanabe SM, et al. Referral criteria for outpatient specialty palliative cancer care: an international consensus. Lancet Oncol. 2016;17:552–559.
- Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–784.
- Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 week of life. Cancer. 2010;116:2716–2725.
- Westhoff PG, de Graeff A, Monninkhof EM, et al. Quality of life in relation to pain response to radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2015;93:694–701.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742.
- Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care interventions on clinical outcomes in patients with advanced cancer: the project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749.
- Zimmerman , et al. Early palliative care for patients with advanced cancer: a cluster-randomized controlled trial. Lancet. 2014;383:1721–1730.
- Hirvonen OM, Alalahti JA, Syrjänen KJ, et al. End-of-life decisions guiding the palliative care of cancer patients visiting emergency department in South Western Finland; a retrospective cohort study. BMC Palliat Care. 2018;17:128.
