Abstract
Introduction: Myelosuppresion is a common side effect of chemotherapy and granulocyte-colony stimulating factor (G-CSF) is often used to reduce the risk of neutropenic events. The purpose of this exploratory analysis was to investigate neutropenic complications in the phase III PANTHER trial of standard 3-weekly chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide plus docetaxel (FEC/D) versus bi-weekly tailored dose-dense EC/D adjuvant chemotherapy in breast cancer.
Patients and methods: Febrile neutropenia, neutropenic infection and infection grade 3–4 according to CTC AE 3.0, were explored in relation to G-CSF use. Per cycle analysis was performed concerning dose reduction and dose delays in conjunction with G-CSF administration.
Results: In the experimental group, 98.9% of patients received primary G-CSF support during EC and 97.4% during docetaxel, compared with 49.7% during FEC and 63.88% during docetaxel in the standard group. Overall, the use of G-CSF was associated with a lower risk for developing neutropenic events (OR 0.44, 95% CI 0.35–0.55, p < .001). Chemotherapy delays due to neutropenia and leukopenia were significantly decreased among patients that received G-CSF (OR 0.098, 95% CI 0.06–0.15 and OR 0.32, 95% CI 0.18–0.58, respectively).
Discussion: In conclusion, G-CSF support reduces neutropenic events and permits increased relative dose intensity, which is essential for improved survival outcomes.
Introduction
Myelosuppression and subsequent neutropenia-related events such as febrile neutropenia or neutropenic infections caused by cytotoxic chemotherapy are major concerns and common dose-limiting toxicities. These complications often require hospitalization, intravenous broad-spectrum antibiotics and occasionally administration of granulocyte-colony stimulating factor (G-CSF), for specific indications such as pneumonia or sepsis [Citation1]. Febrile neutropenia is associated with increased morbidity, mortality and cost [Citation2,Citation3]. Overall in-hospital mortality for cancer patients treated for neutropenic fever is estimated at 2.6% but can be higher in the presence of major co-morbidities [Citation4].
Dose reduction, often but not exclusively subsequent to neutropenic fever or infection, has been correlated with poorer cancer-related outcome in early stage malignancies [Citation3,Citation5,Citation6]. The introduction of G-CSF, either as primary or secondary prophylaxis, has permitted escalation of relative dose intensity without increasing the risk for unacceptable toxicity [Citation7,Citation8]. Current clinical practice guidelines recommend G-CSF as primary prophylaxis for patients with risk of neutropenic fever over 20%, but even patients with intermediate risk (10–20%) should be evaluated for individual risk factors that can increase the overall risk [Citation9–11]. Additionally, patients with neutropenic events during the first chemotherapy cycle should be considered for secondary prophylaxis with G-CSF in subsequent cycles [Citation12]. Notably, in the IMPACT Solid trial most episodes were observed when primary G-CSF prophylaxis was not given [Citation13].
The most commonly used G-CSFs are filgrastim and its polyethylene glycol conjugated form (pegfilgrastim). Long-acting G-CSFs, such as pegfilgrastim, are cleared from the body predominantly by circulating neutrophils whereas short-acting G-CSFs, such as filgrastim, lenograstim and filgrastim biosimilars, can be cleared by the kidneys and therefore require daily doses for a longer period [Citation14]. Pegfilgrastim is considered to be more efficient than filgrastim in terms of reduction of neutropenic complications and duration of hospitalization while simultaneously allowing for achievement of higher relative dose intensity, but is costlier [Citation15–18]. However, there is evidence supporting that pegfilgrastim is the most cost-effective option [Citation19,Citation20].
The PANTHER (Pan-European tailored chemotherapy study) phase III trial investigates the efficacy and safety of tailored according to the hematologic nadirs and dose-dense (tdd) epirubicin (E) and cyclophosphamide (C) followed by tdd docetaxel (D) compared to standard three-weekly epirubicin, cyclophosphamide and 5-fluorouracil (FEC) followed by docetaxel (D) following resection of early breast cancer. In this secondary exploratory toxicity analysis, we aimed to investigate neutropenic- and infection-related complications registered in the study in relation to G-CSF use.
Patients and methods
Study design
The study design and the primary efficacy analysis of the PANTHER randomized phase III multi-centre trial have been previously reported [Citation21]. In short, women with node-positive or high-risk negative, completely resected breast cancer were enrolled in the study. The trial’s primary endpoint was to compare the effect on breast cancer recurrence-free survival of four cycles of two-weekly tdd EC (E 38–120 mg/m2, starting dose 90 mg/m2; C 450–1200 mg/m2, starting dose 600 mg/m2), followed by four cycles tdd D (75–100 mg/m2, starting dose 75 mg/m2) (tdd group) to three cycles of standard interval three-weekly FEC (F 500 mg/m2, E 100 mg/m2, C 500 mg/m2) followed by three cycles of D100 mg/m2 (group B). Both regimens were given with the same duration of 15 weeks. Dose escalation or reduction in group A was performed prior to every chemotherapy cycle in accordance to a predefined algorithm depending on the depth of the hematologic nadirs.
Primary G-CSF prophylaxis was mandatory in the experimental treatment group to ensure that dose-density would be achieved and maintained; both filgrastim or biosimilars and pegfilgrastim were allowed. Filgrastim was administered on days 4–11 during the EC part and days 4–10 during the D part. The same G-CSF schedule was used in the PANTHER phase III study as previously administered in the feasibility phase II study [Citation22]. Pegfilgrastim was administered as a single dose on day 2. Additionally, ciprofloxacin 500 mg b.i.d. was administered, per protocol, as primary prophylaxis on days 5–12 during EC and as secondary prophylaxis during D. No recommendation was initially given for primary G-CSF prophylaxis for the standard treatment group. Secondary prophylaxis was given in the standard treatment group if the neutrophil count on day 21 was <1.5 x109/L or in case of febrile neutropenia events. In summer 2010, following the pre-planned interim analysis for safety and after interaction with the Independent Data Safety and Monitoring Committee, a recommendation was made regarding the policy of G-CSF prophylaxis in the standard treatment group, and as of 18th of June 2010 primary G-CSF prophylaxis was even recommended in the standard group, though not mandatory. Adverse events were registered after each cycle of chemotherapy according to the Common Terminology Criteria (CTCAE) version 3.0 and the highest grade of toxicity in any of the chemotherapy cycles were considered for the summary analysis. Delays and dose reductions in relation to G-CSF administration were considered per cycle. Dose reductions are reported for the standard chemotherapy group since dose reductions according to haematological toxicity were defined per protocol for the tdd chemotherapy group.
All procedures performed in the study were in accordance with the ethical standards of the regional ethics committee and the current laws of Sweden and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The PANTHER study was approved by ethics committees with jurisdiction for the participating sites and the relevant competent authorities (registered at clinicaltrials.gov, identifier NCT00798070). All patients gave a written informed consent to participate in the study.
Statistical methods
An exploratory toxicity analysis was performed using the registered febrile neutropenia and infection grade 3–4 adverse events in the study, in relation to G-CSF use. Randomization date 1st of August 2010 was used as cutoff date for comparison of neutropenic- and infection-related adverse events before and after policy change for primary G-CSF prophylaxis, providing time for the information on policy change to reach the investigators.
Febrile neutropenia (fever over 38.5 °C and absolute neutrophil count [ANC] < 1.0 x109/L, per CTC AE 3.0) or infections with neutropenia (ANC < 1.0 x109/L) were defined as neutropenic events. The use of G-CSF in the standard group was divided according to the clinical indication: administration of G-CSF before febrile neutropenia or neutropenic infection was considered as primary prophylaxis, whereas initiation after a neutropenic event was considered as secondary prophylaxis. Due to differences in the number of given cycles and thus exposure, and the de facto increased risk of haematological toxicity in group A, no statistical comparison was performed regarding neutropenic events between the two groups. Fisher’s exact test was used for comparison of within group differences in the use of G-CSF and incidence of neutropenic events. The analysis was performed using Stata version 14.1 (StataCorp, Lakeway, Texas, USA).
Results
Patient characteristics
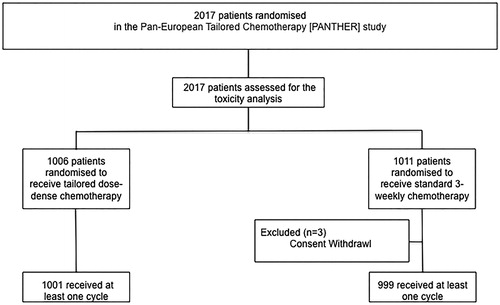
Between July 2007 and September 2011, 2017 patients were randomized in the study; 1006 patients were randomized to receive tdd EC/D (experimental group) and 1011 to standard interval FEC/D (standard group). In this analysis, 2000 patients that received at least one chemotherapy cycle according to protocol have been included (). The median age for group A was 51.1 (range 23.3 to 69.5) and for group B 50.3 (range 21.4 to 68.6) years. Detailed patient characteristics have been previously presented [Citation21].
G-CSF usage
Among the patients within the standard chemotherapy group, 49.7% received G-CSF as primary prophylaxis during FEC and 63.8% during D. Secondary prophylaxis was given to 5.4% during the former and 4.5% during the latter. In the tdd group, the majority (98.9%) received primary prophylaxis during EC and 97.4% during D, with most receiving pegfilgrastim. summarizes the use of G-CSF during chemotherapy according to the intention of the treatment.
Table 1. G-CSF use and type of G-CSF administered according to treatment group; standard interval; versus tailored dose-dense chemotherapy.
Febrile neutropenia, documented infection with neutropenia and documented infection with normal neutrophil counts
In total, 134 (6.7%, 95% CI 5.9–7.9%) patients experienced febrile neutropenia of any grade during the administration of anthracycline-containing regimen and 90 (4.5%, 95% CI 3.7–5.5%) during D. In the standard group, 54 patients (5.4%) had a grade 3 or 4 neutropenic fever event during FEC and 63 patients (6.3%) during D; 16 and 17 patients respectively despite primary prophylaxis. In the experimental group, 70 (7%) patients had a grade 3 or 4 neutropenic fever event during tdd EC and 16 patients (1.6%) during tdd D.
Documented infection with low ANC grade 3 or 4 was reported in 33 (3.3%; 13 had received primary prophylaxis) patients during FEC and 35 (3.5%) patients during tdd EC. The frequency of grade 3 or 4 infection with low ANC was similar in the standard group during D (n = 35, 3.5%; 10 had received primary prophylaxis), whereas it was low during tdd D (n = 8, 0.8%). On the other hand, infection with normal ANC grade 3 or 4 was low during anthracyclines; 30 patients (3%) in group B and 25 (2.5%) in group A. The frequency was similar for the standard group during D (n = 37, 3.7%), although it was numerically higher during tdd D (n = 74, 7.4%).
The risk for neutropenic events (febrile neutropenia or infection with low ANC) grade 3 or 4 in the standard group was significantly reduced after the policy change (Odds Ratio [OR] 0.48, 95% Confidence Interval [CI] 0.31–0.73, p < .001). Importantly, no grade 5 toxicity related to neutropenic events was reported in either of the groups. The use of G-CSF overall was associated with a lower risk for developing neutropenic events (OR 0.44, 95% CI 0.35–0.55, p < .001). The incidence of neutropenic events in relation to G-CSF usage is summarized in .
Table 2. G-CSF use and neutropenic events during epirubicin (both groups combined) and docetaxel (both groups combined).
Exploratory analysis on the efficacy of pegfilgrastim versus filgrastim did not show difference between patients administered G-CSF as primary prophylaxis within the tdd group. During tdd EC 80 (10.20%) patients treated with pegfilgrastim as primary prophylaxis versus 5 (6.02%) treated with filgrastim developed grade 3 or 4 neutropenic events (p = .319). Similarly, 11 (1.46%) versus 4 (3.88%) patients developed grade 3 or 4 neutropenic events during tdd D (p = .095).
Effect of G-CSF usage on compliance to planned treatment
A total of 13434 chemotherapy cycles were administered among the 2000 eligible patients. Of these, 10391 were given with G-CSF support, 2950 without G-CSF support and information on G-CSF administration is missing for 93 cycles. A higher frequency of neutropenic fever grade 3 or 4 (1.21% versus 2.95%) and infection with low ANC events (0.71% versus 1.36%) was documented during cycles without G-CSF support, while infection with normal ANC was more frequent in cycles with G-CSF support (1.52% with G-CSF support versus 0.81% without) (). Infection events regardless ANC were similar with (n = 232, 2.23%) and without (n = 64, 2.17%) G-CSF.
Table 3. Febrile neutropenia, infection with low ANC count and infection with normal ANC with and without G-CSF support.
In both groups, a total of 753 (5.6%) cycles were delayed; 16 due to neutropenic fever, 131 due to infection, 51 due to leukopenia (any grade), 114 due to neutropenia (any grade) and 442 due to other medical or administrative reasons. There was an increased frequency of delays in the standard group (3.3% versus 2.6%) but a formal statistical test was not performed since more cycles were administered in the experimental group as per protocol. The risk of chemotherapy delay due to neutropenia and leukopenia was significantly decreased among patients that received G-CSF (OR = 0.098, 95% CI 0.06–0.15 and OR = 0.32, 95% CI 0.18–0.58, respectively) (). The risk of delay due to neutropenia was decreased by 48% after the policy change (OR = 0.52, 95% CI 0.32–0.84, p < .01) ().
Dose reductions in relation to G-CSF use were analyzed only for the standard group, since they were an integral, protocol-defined part of the experimental tdd treatment. A total of 5705 cycles were administered with planned doses and reduced doses were administered in 165 cycles. The frequency of dose reductions due to neutropenic fever, infection, leukopenia or neutropenia was very low regardless of use of G-CSF.
Discussion
Primary prophylaxis with G-CSF during tdd chemotherapy was feasible in this trial and limited the frequency of grade 3 or 4 febrile neutropenia to 7% or less, which was similar to standard therapy. These results are in accordance with results from a large meta-analysis of randomized trials [Citation23]. Notably, increased frequency of primary prophylaxis in the control group after the policy change reduced neutropenia-related events, as expected, and allowed for improved dose density. The observed absolute reduction of febrile neutropenia by 2.2% in the standard group after change of policy emphasizes the importance of primary prevention in reducing the risk of neutropenic events, which is considered the most cost-effective option [Citation24,Citation25]. This observation is further corroborated by the lower rates of neutropenic complications in the standard group compared with the similar regimen used in the PACS 01 trial [Citation26], presumably due to the lower use of G-CSF support in the latter compared to PANTHER.
Escalating and maintaining escalated dose intensity and density is a critical component of adjuvant chemotherapy for early breast cancer. The theoretical background of this postulate is the Norton–Simon model of cancer cell death being proportional to the tumor growth rate at the time of chemotherapy administration [Citation27]. According to Gompertzian kinetics, tumor cell growth will increase between chemotherapy cycles, meaning that reducing the meantime will result in more effective suppression of tumor regrowth and faster cell-kill [Citation28]. These observations are strongly backed by clinical evidence: lower relative dose intensity than pre-planned doses during adjuvant anthracyclines and taxanes is associated with shorter OS, while the administration of dose-dense chemotherapy improved long term survival compared with standard treatment according to an individual patient data meta-analysis [Citation29,Citation30]. In the PANTHER trial, the next evolutionary step of adjuvant chemotherapy was evaluated [Citation21,Citation31–34], since dose tailoring may overcome the significant inter-patient variability in terms of pharmacokinetics. This analysis demonstrates the feasibility of such an approach and that G-CSF primary prophylaxis clearly allows for increased relative dose intensity while simultaneously limiting the risk of neutropenic complications.
Limitations of this study that need to be acknowledged include the initial lack of recommendation for primary G-CSF support in the standard group. This could introduce bias, since primary prophylaxis with G-CSF was not standardized across participating centers but was administered in accordance to clinical guidelines, local practice and physician’s choice [Citation35]. In addition, the relative contribution of prophylactic ciprofloxacin in the reduction of infectious complications in the experimental group should also be taken into consideration when interpreting the results of this study. Moreover, within group comparison of the efficacy of pegfilgrastim and filgrastim in preventing neutropenic events in the tdd group did not show any difference, in contrast to previous reports [Citation36]. However, these results should be interpreted with caution since the population for the analysis was not randomized. Further limitations are consistent with the exploratory nature of the analysis and lack of a pre-planned power calculation to identify statistical differences.
In conclusion, primary prophylaxis with G-CSF reduces the incidence of neutropenia-related events and G-CSF support was shown in our trial to be both feasible and effective, allowing also for dose-tailoring and increased dose intensity without excess myelotoxicity.
Author contributions
AP and HJ performed the analysis. MH and HJ were responsible for data acquisition and data preparation. AP and AM prepared drafting of the manuscript. AP prepared all figures and tables of the manuscript. AM and JB were responsible for the overall supervision of the project and JB also for the funding of the project. All authors critically reviewed the manuscript.
| Abbreviations | ||
| ANC | = | absolute neutrophil count |
| CI | = | confidence interval |
| CTC AE | = | common terminology criteria for adverse events |
| D | = | docetaxel |
| FEC | = | 5-fluorouracil – epirubicin – cyclophosphamide |
| G-CSF | = | Granulocyte-Colony stimulating factor |
| OR | = | odds ratio |
| RDI | = | relative dose intensity |
| tdd D | = | tailored and dose dense docetaxel |
| tdd EC | = | tailored and dose dense epirubicin and cyclophosphamide |
Supplemental Material
Download MS Word (76.3 KB)Disclosure statement
Antroula Papakonstantinou, Mats Hellström, Hemming Johansson, Michael Untch and Alexios Matikas have no conflicts of interest to disclose. Elham Hedayati research fund from Roche. Michael Gnant: Employment (Sandoz) of an immediate family member; Honoraria from Amgen, AstraZeneca, Celgene, EliLilly, Invectys, Pfizer, Nanostring, Novartis, Roche, and Medison; Consulting for AstraZeneca and EliLilly; Travel support from Amgen, AstraZeneca, EliLilly, Ipsen, Pfizer, Roche, Medison. Guenther Steger: personal fees and nonfinancial support from Amgen; Richard Greil: honoraria from Amgen, Roche, AstraZeneca and MSD; consulting role in Celgene, Roche, BMS and AstraZeneca; travel fees from Roche, Amgen, AstraZeneca, Janssen; research funding from Celgene, Roche, BMS and AstraZeneca; Volker Moebus: horonaria from Amgen, Celgene, Roche, Myelotherapeutics and AstraZeneca; advisory role at Myelotherapeutics, Roche and Amgen personal fees from Amgen, Celgene, Roche and AstraZeneca; Sibylle Loibl: honoraria to institution from AstraZeneca; Amgen, Pfizer, Roche, Puma, Novartis; consulting role in AstraZeneca, Amgen, Novartis, Pfizer, Roche, Puma; research funding to institution from Amgen, Astra Zeneca, Celgene, Novartis, Pfizer, Roche, Teva, Vifor; Theodoros Foukakis: institutional grants from Roche and Pfizer and personal fees from Novartis, Pfizer, Roche and UpToDate; Jonas Bergh: research fundings from Merck paid to Karolinska Institutet and from Amgen, Bayer, Pfizer, Roche and Sanofi-Aventis paid to Karolinska University Hospital. No personal payments. Payment from UpToDate for a chapter in breast cancer prediction paid to Asklepios Medicine HB.
Additional information
Funding
References
- NCCN. Prevention and treatment of cancer-related infections. NCCN.org; [cited 2018 Dec 17].
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266.
- Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99–108.
- Pathak R, Giri S, Aryal MR, et al. Mortality, length of stay, and health care costs of febrile neutropenia-related hospitalizations among patients with breast cancer in the United States. Support Care Cancer. 2015;23(3):615–617.
- Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332(14):901–906.
- Colleoni M, Li S, Gelber RD, et al. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366(9491):1108–1110.
- Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167.
- Leonard RC, Mansi JL, Keerie C, et al. A randomised trial of secondary prophylaxis using granulocyte colony-stimulating factor ('SPROG' trial) for maintaining dose intensity of standard adjuvant chemotherapy for breast cancer by the Anglo-Celtic Cooperative Group and NCRN. Ann Oncol. 2015;26(12):2437–2441.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
- Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10(6):427–437.
- Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27(suppl_5):v111–v8.
- Chan A, McGregor S, Liang W. Utilisation of primary and secondary G-CSF prophylaxis enables maintenance of optimal dose delivery of standard adjuvant chemotherapy for early breast cancer: an analysis of 1655 patients. Breast. 2014;23(5):676–682.
- Maenpaa J, Varthalitis I, Erdkamp F, et al. The use of granulocyte colony stimulating factor (G-CSF) and management of chemotherapy delivery during adjuvant treatment for early-stage breast cancer-further observations from the IMPACT solid study. Breast. 2016;25:27–33.
- Yang BB, Savin MA, Green M. Prevention of chemotherapy-induced neutropenia with pegfilgrastim: pharmacokinetics and patient outcomes. Chemotherapy. 2012;58(5):387–398.
- Siena S, Piccart MJ, Holmes FA, et al. A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily Filgrastim in patients with stage II-IV breast cancer. Oncol Rep. 2003;10(3):715–724.
- Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Pract. 2016;22(5):702.
- Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045–2051.
- Pfeil AM, Allcott K, Pettengell R, et al. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer. 2015;23(2):525–545.
- Danova M, Chiroli S, Rosti G, et al. Cost-effectiveness of pegfilgrastim versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori. 2009;95(2):219–226.
- Perrier L, Lefranc A, Perol D, et al. Cost effectiveness of pegfilgrastim versus filgrastim after high-dose chemotherapy and autologous stem cell transplantation in patients with lymphoma and myeloma: an economic evaluation of the PALM Trial. Appl Health Econ Health Policy. 2013;11(2):129–138.
- Foukakis T, von Minckwitz G, Bengtsson NO, et al. Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: a randomized clinical trial. JAMA. 2016;316(18):1888–1896.
- Margolin S, Bengtsson NO, Carlsson L, et al. A randomised feasibility/phase II study (SBG 2004-1) with dose-dense/tailored epirubicin, cyclophoshamide (EC) followed by docetaxel (T) or fixed dosed dose-dense EC/T versus T, doxorubicin and C (TAC) in node-positive breast cancer. Acta Oncol. 2011;50(1):35–41.
- Renner P, Milazzo S, Liu JP, et al. Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev. 2012;10:CD007913.
- Lee EK, Wong WW, Trudeau ME, et al. Cost-effectiveness of prophylactic granulocyte colony-stimulating factor for febrile neutropenia in breast cancer patients receiving FEC-D. Breast Cancer Res Treat. 2015;150(1):169–180.
- Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin's lymphoma: cost-effectiveness analyses. J Med Econ. 2014;17(1):32–42.
- Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24(36):5664–5671.
- Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Rev Clin Oncol. 2006;3(8):406–407.
- Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol. 1997;24(4 Suppl 10):S10.
- Early Breast Cancer Trialists' Collaborative G. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393(10179):1440–1452.
- Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444.
- Poikonen P, Saarto T, Lundin J, et al. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer. 1999;80(11):1763–1766.
- Matikas A, Margolin S, Hellstrom M, et al. Long-term safety and survival outcomes from the Scandinavian Breast Group 2004-1 randomized phase II trial of tailored dose-dense adjuvant chemotherapy for early breast cancer. Breast Cancer Res Treat. 2018;168(2):349–355.
- Cameron DA, Massie C, Kerr G, et al. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer. 2003;89(10):1837–1842.
- Matikas A, Foukakis T, Moebus V, et al. Dose tailoring of adjuvant chemotherapy for breast cancer based on hematologic toxicities: further results from the prospective PANTHER study with focus on obese patients. Ann Oncol. 2019;30:109–114.
- Crawford J, Caserta C, Roila F, et al. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010;21(Supplement 5):v248–51.
- von Minckwitz G, Kummel S, Du Bois A, et al. Pegfilgrastim +/- ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol. 2008;19(2):292–298.