Introduction
Regenerative nodular hyperplasia (RNH) is an uncommon side effect of trastuzumab entamsine (T-DM1). To our knowledge, 11 cases of histologically proven RNH have been described, most of the time after a year or more of T-DM1 treatment [Citation1–4]. We describe the only case of pathologically confirm early stage RNH and discuss the importance of knowing this side effect and of its early diagnosis.
Case 1
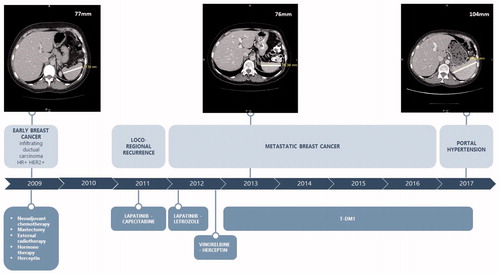
The first patient is a 52-year-old woman, with no significant medical history, who was initially diagnosed in 2009 with HER2-positive, hormone receptor-positive, inflammatory infiltrating ductal carcinoma on the right breast, and staged pT2N1M0. She was treated with neoadjuvant chemotherapy (3 cycles of 5-Fluorouracil – Epirubicin – Cyclophosphamide followed by 3 cycles of Docetaxel) with Trastuzumab for one year, mastectomy and axillary lymph node dissection, external radiotherapy, and 5 years of Tamoxifen. In 2011, she relapsed with brain and skin metastases, and received successively between 2011 and 2013 Lapatinib – Capecitabine, Lapatinib – Letrozole, Trastuzumab – Vinorelbine. In April 2013, she experienced disease progression with new skin metastases. Therefore, T-DM1 was initiated. Baseline biological tests were within normal range and a baseline abdominal computed tomography (CT) scan revealed normal-appearing liver with no evidence of portal hypertension. In July 2014 after 23 cycles of T-DM1 appeared an asymptomatic grade 1 liver enzymes increase (AST and ALT <2 N, grade 1 according to CTCAE v5) with anicteric cholestasis (ALP (alkaline phosphatase) < 2 N, GGT = 23 N, normal total bilirubin). These biological tests remained stable from June 2014 to April 2017 without dose reduction or treatment interruption. Abdominal CT scans, which were realized for disease’s follow up, did not show liver changes. In April 2017 after 68 cycles of T-DM1, grade 1 thrombocytopenia (=102 G/L) and grade 1 bilirubin increase (total bilirubin = 1.5 N, conjugated bilirubin = 1.3 N) appeared. Abdominal ultrasound showed a discretely heterogenous liver echo texture. Transient elastography found moderate fibrosis. Upper gastrointestinal endoscopy showed a mosaic like pattern in the gastric mucosa leading to the diagnosis of portal hypertension gastropathy without esophageal varices. Portal hypertension with splenomegaly and venous collateral circulation were described on the abdominal CT scan (). Liver biopsy () showed centrilobular sinusoidal dilatation and congestion with perisinusoidal fibrosis and congestion constituting focally bridging between central veinules. Atrophic hepatic plates alternated with thickened plates. Portal tracts were normal with neither intraportal inflammation nor interface hepatitis. Reticulin stain showed partial parenchyma nodular transformation. A diagnosis of non cirrhotic portal hypertension as a result of partial nodular regenerative hyperplasia was established. Following this diagnosis, T-DM1 was discontinued and switched to Trastuzumab – Letrozole. The patient has not yet needed any treatment for her portal hypertension. In Mars 2019, liver dysfunction appears stable with no improvement or worsening since RNH diagnosis.
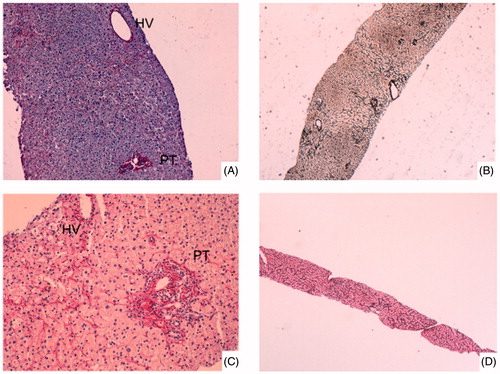
Figure 2. A - Case #1: Portal tracts (PT) and hepatic vein (HV) were normally organized. Atrophic hepatic plates alternated with thickened plates (Picro-hemalun stain, original magnification × 100). B - Case #1: Reticulin stain showed parenchyma nodular transformation (Gordon Sweet reticulin stain, original magnification × 40). C - Case #2: Portal tracts (PT) and hepatic vein (HV) were normally organized. There is congestion with perisinusoidal fibrosis in centrolobular areas (Picro-hemalun stain, original magnification × 100). D - Case #2: Reticulin stain did not showed nodular transformation of liver parenchyma (Gordon Sweet reticulin stain, original magnification × 10).
Case 2
The second patient is a 48-year-old woman, with no significant medical history, who was initially diagnosed in 2009 with HER2-positive, hormone receptor-positive, infiltrating ductal carcinoma on the right breast, with de novo liver and bone metastases. Between 2009 and 2013, she received several lines of treatment because of multiple bone progressions: Docetaxel – trastuzumab followed by endocrine therapy with Triptorelin - Tamoxifen, then Triptorelin – Letrozole, and again docetaxel followed by endocrine therapy with Triptorelin – Fulvestrant and Trastuzumab
T-DM1 was initiated in January 2013 due to locoregional progression. Baseline biological tests were within normal range and baseline abdominal CT scan showed no evidence of liver disease (metastasis or portal hypertension). In July 2013 after 8 cycles, an asymptomatic grade 1 transaminases increase (AST = 3 N, ALT <2 N) with a grade 1 anicteric cholestasis (ALP < 2 N, GGT = 7 N, total bilirubin = 1.3 N) was described. In December 2013, grade 1 bilirubin increase (total bilirubin = 1,5 N, conjugated bilirubin = 2.3 N) appeared concomitantly with grade 1 thrombocytopenia (=103 G/L). Hepatic tests remained stable with grade 1 elevated transaminases and bilirubin (AST <2 N, ALT normal, ALP < 2 N, GGT = 13 N, total bilirubin = 1.3 N). In October 2017, TDM-1 was stopped after 77 cycles and switched to Lapatinib – Capecitabine because of bone progression. In November 2017, patient experienced lower limbs edema associated with ascites. Laboratory tests showed cholestasis (GGT 3 N, ALP <2 N, total bilirubin = 1.8 N, conjugated bilirubin = 4 N) but no transaminases increase. Cardiac physical examination, NT-proBNP and serum creatinine were normals, excluding the hypothesis of a cardiac or renal cause. Abdominal CT scan described heterogenous liver and splenomegaly. Liver biopsy () revealed centrilobular sinusoidal dilatation and congestion with perisinusoidal fibrosis. Unless atrophy of hepatic plates in the congestive areas, this was no other alteration in the thickness of hepatic plates. Reticulin stain did not showed nodular transformation of liver parenchyma. Portal tracts were normal with no extensive fibrosis. A diagnosis of non-cirrhotic portal hypertension as a result of sinusoidal obliterative syndrome – like changes was established. Symptoms successfully resolved with Aldactone administration. No long-term treatment was established for her portal hypertension. Capecitabine and lapatinib were continued with no further complication. In Mars 2019, the patient remains free of ascites or edema with stable grade 1 bilirubin elevation and thrombocytopenia.
Discussion
Amplification of HER2 occurs in approximately 15% of breast cancer. Trastuzumab emtansine or T-DM1 is an antibody-drug conjugate: chemotherapy DM1, spindle poison inhibiting microtubule polymerization, linked to a humanized anti-HER2 monoclonal antibody. In 2012, the pivotal phase 3 EMILIA study demonstrated the superiority of T-DM1 (versus lapatinib plus capecitabine) in second line therapy for patients with HER-positive advanced breast cancer. Median progression-free survival was 9.6 months with TDM-1 (versus 6,4 with lapatinib plus capecitabine; hazard ratio 0.65; p < .001) and overall survival was 30,. months (versus 25,1; hazard ratio 0.68; p < .001) [Citation5]. A second phase III study THERESA demonstrated also the superiority of T-DM1 (versus physician’s choice) on population which have received at least 2 lines including taxane, anthracycline or lapatinib. Median progression-free survival was 6.2 months with TDM-1 (versus 3,3; hazard ratio 0.528; p < .0001) and median overall survival was 22.7 months (vs 15·8 months; hazard ratio 0.68; p = .0007) [Citation6,Citation7].
In clinical trials, transaminases elevation was one of the most common grade 3 or 4 adverse events with TDM-1 (increased AST (4.2% in EMILIA and 2% in THERESA) and increased ALT (2.9% and 2%, respectively)). Elevated transaminases of any grades were 22.4% (AST) and 16.9% (ALT) and 12% (AST) and 10% (ALT), respectively. Later on, this adverse event has been reported in all studies as a common adverse event of TDM1. However, it was not considered as a serious adverse event because of the spontaneous improvement of biologic liver function in most cases after dose reduction with possibility to resume treatment [Citation8,Citation9].
RNH seems to be caused by DM1 [Citation10,Citation11]. DM1 is a maytansine derivative. Maytansine has a potent cytotoxic effects [Citation12,Citation13] but acceptable therapeutic indices were not obtained [Citation14–16]. Toxicity of DM1 can be explained by its hepatic metabolism and by its elimination that happens mostly in the biliary system [Citation17,Citation18]. Based on the pharmacokinetics’ results of 3 studies in which correlations between T-DM1 exposure (area under the curve, maximum concentration and minimum concentration) and levels of AST and ALT were assessed, no obvious relationship was observed between exposure and changes in serum concentrations of AST or ALT. There is no link found either between exposure and grade 3 increases in AST or ALT [Citation19]. The cases described here and others described in the literature seem to show the importance of the duration of exposure.
The chronological association between TDM1 exposure and the appearance of RNH suggests a cause-effect association. Potential other causes have been excluded.
RNH is characterized by micronodularity of the liver without fibrosis. This lesion appears as small nodules (less than 0.2 cm) of normal or hyperplastic plates of hepatocytes adjacent to strands of atrophic compressed trabeculae. The nodularity is best shown on a reticulin stain. The pathogenesis of RNH is related to changes of intrahepatic blood flow, leading to atrophic hypoperfused areas intermingled with hyperperfused regenerative areas. It is currently believed than the circulatory impairment can start either as obstructive portal vein injury [Citation20] or at the level of sinusoids [Citation21,Citation22]. Drug-induced RNH is not usually associated with obliterative portal veinopathy. One of our case showed isolated centrilobular sinusoidal lesions with no regenerative liver nodules and was interpreted as sinusoidal obstruction syndrome – like changes. This suggests that non-cirrhotic portal hypertension associated with T-DM1 could initially result from sinusoidal endothelium damages. Sinusoidal obstruction syndrome is characterized by sinusoidal endothelium damage with or without occlusion of the central vein resulting in sinusoidal congestion and dilatation. Sinusoidal obstruction syndrome exclusively occurs namely in a context of exposure to toxic agents for liver sinusoidal endothelium. Sinusoidal obstruction syndrome has been shown to give rise to RNH [Citation23]. Hence, RNH could be an evolutive process in which regenerative nodules progressively spread liver. Therefore, it is possible that in one of our patients with non-cirrhotic portal hypertension, liver biopsy missed such locally and haphazardly distributed regenerative nodule. Two lines of evidence advocate for prior occurrence of sinusoidal obliterative syndrome to RNH: (i) T-DM1 could internalized into Fc-receptor-bearing Kupffer cells with subsequent release free T-DM1 after Kupffer cells destruction in the microenvironment; (ii) failure of bone marrow progenitor to repopulate sinusoids with endothelial cells is instrumental to cause disease [Citation24]. Such situation is frequent in patients with metastatic disease having received multiple lines of chemotherapy. Finally it is interesting to note that one of the patient reported by Force et al. [Citation3] received FU and oxaliplatine before T-DM1 regimen. Indeed, oxaliplatin-based chemotherapy in patients with colorectal liver metastasis is known to be associated with RNH due to the formation of Sinusoidal obstruction syndrome [Citation25]. In our 2 cases, among the drugs our patients previously received, only cyclophosphamide has been described in one case report as a possible cause of RNH [Citation26]. One of our cases shows that sinusoidal obliterative syndrome might be related to TDM-1 and lead to RNH. To our knowledge, this is the first reported case of TDM-1 associated with sinusoidal obliterative syndrome as a potential first step in RNH development.
With these cases, we want to stress that the major issue is to detect and diagnose RNH as early as possible. Therefore, elevations in transaminase levels must be closely monitored.
RNH impact is not on mortality but on quality of life with a risk of appearance of portal hypertension in patients with advanced cancer. In this population, one of the main goal for treatment is quality of life [Citation27]. Von Minckwitz and al. showed recently among patients with HER2-positive early breast cancer who had residual invasive disease after completion of neoadjuvant therapy, the risk of recurrence of invasive breast cancer or death was 50% lower with adjuvant T-DM1 than with trastuzumab alone [Citation28]. In this study, elevated transaminases of any grades were 28.4% (AST) and 23.1% (ALT) with 1.6% and 1.5% of grade 3 or higher, respectively. On May 3, 2019, the Food and Drug Administration approved T-DM1 for the adjuvant treatment of patients with HER2-positive early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment based on that study, whereas it is well specified that RNH should be considered in all patients with clinical symptoms of portal hypertension and/or cirrhosis-like pattern seen on the computed tomography scan of the liver.
Thus, we have to better understand this side effect in order to detect it as early as possible; i.e before the appearance of clinical signs. Indeed, cases of RNH regression after discontinuation of T-DM1 seem to have been previously described [Citation29]. Diagnosis of RNH should lead to definitive discontinuation of this drug.
References
- Diéras V, Harbeck N, Budd GT, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. JCO. 2014;32(25):2750–2757.
- Prochaska LH, Damjanov I, Ash RM, et al. Trastuzumab emtansine associated nodular regenerative hyperplasia: a case report and review of literature. Cancer Treat Commun. 2016;5:26–30.
- Force J, Saxena R, Schneider BP, et al. Nodular regenerative hyperplasia after treatment with trastuzumab emtansine. JCO. 2016;34(3):e9–12.
- Lepelley M, Allouchery M, Long J, et al. Nodular regenerative hyperplasia induced by trastuzumab emtansine: role of emtansine? Ann Hepatol. 2018;17(6):1067–1071.
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791.
- Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. Jun
- Krop IE, Kim S-B, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–754.
- Shen K, Ma X, Zhu C, et al. Safety and efficacy of trastuzumab emtansine in advanced human epidermal growth factor receptor 2-positive breast cancer: a Meta-analysis. Sci Rep. 2016;6(1):23262.
- Kowalczyk L, Bartsch R, Singer CF, et al. Adverse events of trastuzumab emtansine (T-DM1) in the treatment of HER2-positive breast cancer patients. Breast Care Basel Switz. 2017;12(6):401–408.
- Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. JCO. 2010;28(16):2698–2704. 1
- Amiri-Kordestani L, Blumenthal GM, Xu QC, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(17):4436–4441. 1
- Remillard S, Rebhun LI, Howie GA, et al. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189(4207):1002–1005. 19
- Wolpert-Defilippes MK, Adamson RH, Cysyk RL, et al. Initial studies on the cytotoxic action of maytansine, a novel ansa macrolide. Biochem Pharmacol. 1975;24(6):751–754.
- Blum RH, Kahlert T. Maytansine: a phase I study of an ansa macrolide with antitumor activity. Cancer Treat Rep. 1978;62(3):435–438.
- Phase II trials of maytansine, low-dose chlorozotocin, and high-dose chlorozotocin as single agents against advanced measurable adenocarcinoma of the pancreas. Gastrointestinal Tumor Study Group. Cancer Treat Rep. 1985;69(4):417–420.
- Franklin R, Samson MK, Fraile RJ, et al. A phase I-II study of maytansine utilizing a weekly schedule. Cancer. 1980;46(5):1104–1108.
- Shen B-Q, Bumbaca D, Saad O, et al. Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (T-DM1): an emphasis on preclinical and clinical catabolism. CDM. 2012;13(7):901–910.
- Shen B-Q, Bumbaca D, Yue Q, et al. Non-clinical disposition and metabolism of DM1, a component of trastuzumab emtansine (T-DM1), in sprague dawley rats. DML. 2015;9(2):119–131.
- Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229–1240.
- Wanless IR, Godwin TA, Allen F, et al. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980;59(5):367–379.
- DeLeve LD. Hepatic microvasculature in liver injury. Semin Liver Dis. 2007;27(4):390–400. Nov
- Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75(4):225–230.
- Rubbia-Brandt L, Lauwers GY, Wang H, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56(4):430–439.
- Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137(2):704–712.
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2004;15(3):460–466.
- Xue D-Q, Yang L. Development of focal nodular hyperplasia after cyclophosphamide-based chemotherapy in a patient with breast cancer. Case Rep Hepatol. 2018;2018:5409316.
- Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. WJG. 2011;17(11):1400–1409.
- von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628.
- Ghabril M, Vuppalanchi R. Drug-induced nodular regenerative hyperplasia. Semin Liver Dis. 2014;34(2):240–245.