Background
In the past decades, when dacarbazine-based chemotherapy was the treatment of choice, the median survival of patients with unresectable melanoma was only six to nine months and only 10–15% of the patients were alive after three years [Citation1]. Dacarbazine as single agent demonstrated an objective response of 13% with a median overall survival (OS) ranging from 5.6 to 11 months according to eight randomized studies, and a one-year OS was 20–30% in five randomized studies [Citation2]. Combination chemotherapy and interferon-alpha (IFN) increased response rate to 50–60% [Citation3–5] but failed to prolong survival [Citation1]. The median OS of 8.9 months was observed with dacarbazine, lomustine and vincristine ± IFN in a retrospective analysis of Finnish melanoma patients [Citation6]. Immune checkpoint inhibitors, v-Raf murine sarcoma viral oncogene homolog B (BRAF), and mitogen-activated protein kinase (MEK) inhibitors have dramatically improved the survival of metastatic melanoma patients: three-year OS rate has reached 44–58% in recent trials with BRAF + MEK inhibitors and immune checkpoint inhibitors [Citation7–9]. Brain metastases are still associated with poor survival, although remarkably long intracranial responses have been observed with combination therapies: 12-month progression-free survival (PFS) rate of 19–47% with dabrafenib + trametinib and 9-month PFS rate of 56.6% with ipilimumab + nivolumab [Citation10,Citation11].
Material and methods
Study design
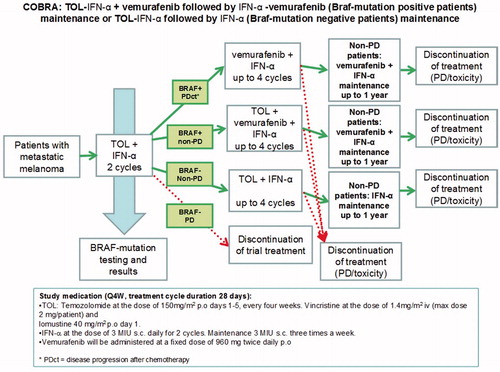
The study was an open-label, two-cohort trial initiated by the Finnish Melanoma Group. It was conducted at the University Hospitals of Helsinki, Turku, Tampere, Kuopio and Oulu. Our objective was to evaluate the efficacy and safety of chemoimmunotherapy (temozolomide, vincristine, lomustine q4w plus subcutaneous interferon-alpha; TOL-IFN) ± vemurafenib in patients with unresectable, advanced, melanoma with no prior therapy for advanced disease, except for immunotherapy. Patients with brain metastases were eligible and intracranial disease progression was evaluated. Eligibility criteria are shown in . The first treatment in the study began in February 2014. The follow-up cut off was in September 2019. Cohort 1 included BRAFv600-positive patients and cohort 2 included BRAFv600-negative patients. BRAF-mutation was analyzed from the primary tumor or metastasis with next-generation-gene sequencing or fully integrated, real-time polymerase chain reaction (PCR)-based IdyllaTM system.
Table 1. Patient characteristics.
The primary objective was to evaluate the response rate after two cycles of TOL-IFN (ORR1) and after further study therapy (ORR2). Secondary objectives included OS, PFS, OS of the patients with baseline brain metastases, intracranial PFS and safety. TOL-IFN + vemurafenib combination was considered successful, if ORR of at least 30% was reached in the study with acceptable toxicity. The response was evaluated according to Recist 1.1 criteria [Citation12] with thoracic and abdominal CT every two cycles (q8 weeks) during TOL-IFN therapy, continuing every 12 weeks during maintenance therapy until disease progression or treatment discontinuation. Brain CT or MRI was used to evaluate patients with brain metastases. Adverse events were reported according to CTCAE version 4.03 [Citation13].
A flow chart of the study treatment is shown in . All patients received two cycles of TOL-IFN: temozolomide 150 mg/m2 p.o. (maximum of 300 mg) days 1–5, vincristine 1,4mg/m2 i.v. (maximum of 2 mg) day 1, lomustine 40 mg/m2 p.o. (maximum of 80 mg) day 1 + IFN-alpha-2a (3MIU s.c. daily for the first 2 cycles and 3MIU 3 times per week thereafter) every four weeks. Vemurafenib 960 mg p.o. twice a day (BID) was added to the treatment of BRAF-positive patients in cohort 1 after two cycles of TOL-IFN. A maximum of six cycles of TOL-IFN were administered. Maintenance therapy contained vemurafenib + IFN in cohort 1 or IFN only in cohort 2. During TOL-IFN therapy dexametason 9 mg p.o. and granisetron 2 mg p.o. were given before vincristine and lomustine in day 1 and metoclopramide 10 mg and granisetron 1 mg p.o. if needed (PRN) thereafter for antiemetic therapy. Laxatives (e.g. lactulose and sodium picosulfate PRN) were recommended to prevent constipation. Careful sun protection instructions were given for BRAF-positive patients before vemurafenib therapy.
The results of continuous variables were presented as median (range) and categorical variables as number and percentages. OS was measured from the day of the first TOL-IFN to death or the last day of the follow-up in September 2019. PFS and intracranial PFS were calculated similarly to the radiological disease progression or the last day of the follow-up. Kaplan–Meier curves were used to illustrate the differences in PFS and OS. Kaplan–Meier estimates were presented with 95% confidence intervals (95% CI) and statistical differences between survival curves were compared using Log Rank test. Pearson 2-sided Chi-Square test was used to compare differences in ORR between treatment cohorts.
Results
Patient characteristics
Thirty-eight patients with metastatic cutaneous melanoma were treated within the study between 2014 and 2019. Although prior immunotherapy was accepted, all patients were treatment naïve in this study. Cohort 1 included 14 patients with BRAFV600-mutation and cohort 2 included 24 BRAF V600-negative patients. Patient characteristics are shown in . There were more patients with lower performance status ECOG 2 (17% vs. 7%), elevated LDH (75% vs. 50%) and baseline brain metastases (29% vs. 14%) in cohort 2 compared to cohort 1.
Treatment
The most common number of TOL-IFN cycles was two in both cohorts and 71% of the patients had discontinued TOL-IFN because of disease progression (PD) after 2 cycles of TOL-IFN in both cohorts. In cohort 1 two patients (14%) received one cycle, eight patients (57%) received two cycles, one patient (7%) received five cycles and three patients (21%) completed all six cycles of TOL-IFN. In cohort 2 four patients (17%) received one cycle, 13 patients (54%) received two cycles, two patients (8%) received four cycles, one patient (4%) received five cycles and four patients (17%) completed all six cycles of TOL-IFN. The median duration of IFN was 2.3 months (0.7–20.1) in cohort 1 and 1.1 months (0.5–17.3) in cohort 2. The median duration of vemurafenib was 4.3 (0–25.5) months in cohort 1. Two patients (14%) did not get vemurafenib due to rapid PD. Four patients (29%) received TOL-IFN + vemurafenib combination. All vemurafenib therapies had been discontinued in September 2019.
Response to therapy
After two cycles of TOL-IFN ORR1 was 14% (one complete response (CR) and one partial response (PR)) in cohort 1 and 8% (two PRs) in cohort 2. There was no statistically significant difference in ORR1 between cohort 1 and 2 (Pearson 2-sided Chi-Square p = .564). The response rate for further therapy ORR2 was 57% (three CRs and five PRs) in cohort 1 after adding vemurafenib to the treatment and 13% (one CR and two PRs) in cohort 2 after continuing TOL-IFN and the difference in ORR2 between cohort 1 and 2 was statistically significant (Pearson 2-sided Chi-Square p = .003). Twelve patients (86%) developed PD in cohort 1: six (43%) received BRAF + MEK inhibitors, three (21%) PD-1 inhibitors and four (29%) chemotherapy after PD. 23 patients (96%) developed PD in cohort 2: six (25%) received PD-1 or CTLA-4 inhibitors and eight (33%) chemotherapy after PD.
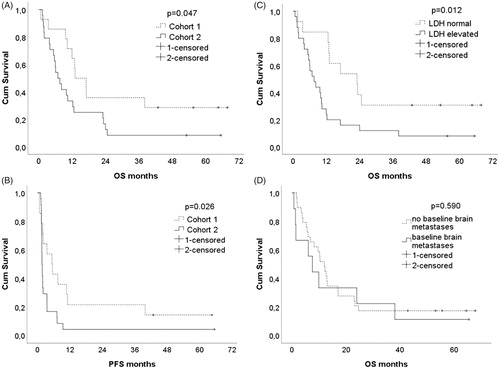
After the median follow-up of 10.3 months (range 0.7–67.8 months), the median overall survival (OS) was 15.1 months (0.7-67.8) in cohort 1 and 7.2 months (1.3-65.5) in cohort 2 and the difference in OS between treatment cohorts was statistically significant (Log Rank p = .047). The median PFS was 5.5 months (0.7–64.5) in cohort 1 and 1.7 months (1.2-65.5) in cohort 2 and the difference in PFS was statistically significant (Log Rank p = .019). The Kaplan–Meier survival curves of OS and PFS are shown in . Six patients (16% of all patients), four BRAF-positive (29% of cohort 1) and two BRAF-negative (8% of cohort 2), were alive in September 2019. Elevated baseline LDH was an adverse prognostic marker for survival: estimated median OS of patients with elevated baseline LDH was 7.6 months (95% CI 4.1–11.0 months) compared to 23.1 months (95% CI 11.0–35.1 months) in patients with normal baseline LDH (Log Rank p = .012) (.
OS of patients with baseline brain metastases and intracranial PFS
Nine out of 38 patients (24%) had baseline brain metastases; two out 14 (14%) patients in cohort 1 and seven out of 24 (29%) patients in cohort 2. The Kaplan–Meier estimate of the OS of patients with baseline brain metastases was not statistically significantly shorter compared to patients without baseline brain metastases (median OS 7.6 months (95% CI 3.2–11.9) versus 12.0 months (95% CI 8.5–15.4), Log rank p = .590), . Two patients with baseline BRAF-negative brain metastases had unexpectedly long survival (24 months and +65.5 months). Both of them had only one brain metastasis and the latter patient had got stereotactic radiotherapy. Radiological progression of brain metastases was discovered in three patients (33%) with baseline brain metastases. New brain metastases were found in 15 patients (52%) without baseline brain metastases. Time to intracranial disease progression was not statistically significantly different between patients with or without baseline brain metastases: estimated median time to intracranial disease progression 9.5 months (95% CI 5.7–13.3) vs. 15.0 months (95% CI 7.7–22.3) (Log Rank p = .776).
Dose reductions and adverse effects
Temozolomide dose was reduced in 21% and 8%, vincristine in 29% and 4% and lomustine dose in 64% and 63% of patients in cohort 1 and 2, respectively. Only two patients experienced grade 3–4 infection (one in both cohorts) during chemotherapy. The most common adverse events (AE) during chemotherapy in both cohorts were fatigue (16%, five patients grade 1–2, one patient grade 3), decreased platelet count (11%, four patients grade 1–2) and constipation (11%, three patients grade 1–2, one patient grade 3). IFN was discontinued due to adverse events in 64% and 13% of patients in cohort 1 and 2, respectively. The most common reasons were rash 8%, infection 8% and fatigue 5%.
Vemurafenib therapy was started 960 mg BID per study protocol. Nine out of twelve patients (75%) had dose reductions and six patients (50%) discontinued vemurafenib due to AE. Four patients (33%) discontinued vemurafenib because of AEs affecting skin: three patients developed grade 3 rash (25%) and one patient experienced intolerable photosensitivity (8%). One patient discontinued vemurafenib due to grade 2 QT-prolongation (8%) and one due to retinopathy (8%). Four patients received TOL-IFN + vemurafenib combination. One of them discontinued vemurafenib after six days due to grade 3 rash and three patients received four TOL-IFN cycles with vemurafenib. All of them had vemurafenib dose reduced to 480 mg BID during the combination therapy.
Discussion
The present study was initiated to analyze the efficacy and safety of chemoimmunotherapy with vemurafenib in advanced melanoma patients including patients with brain metastases. In this study, TOL-IFN reached a similar 13–14% response rate that has been observed earlier with dacarbazine-based chemotherapy [Citation1]. The toxicity of TOL-IFN could be managed with dose reductions, antiemetics and laxatives. After adding vemurafenib to the treatment of BRAF-positive patients the ORR, PFS and OS increased significantly, as expected. The combination of vemurafenib with TOL-IFN could be given with acceptable toxicity after vemurafenib dose reductions. The present study was too small to recommend TOL-IFN + vemurafenib combination outside clinical trials.
The limitations of this study include small numbers of patients. Vemurafenib was provided by Roche for BRAF-positive patients in the study before it was reimbursable in Finland. One year after the initiation of this study BRAF + MEK inhibitors (dabrafenib + trametinib and vemurafenib + cobimetinib) became available, which made it difficult to recruit BRAF-positive patients into the study. Therefore, it was decided to close the recruitment of the study before the preplanned patient number of 48 patients was reached. This made imbalance between cohort 1 and 2, with more favorable baseline characteristics in the BRAF-positive cohort 1, which could have had an effect on the efficacy of the study therapy and caused bias to our study. The use of immune checkpoint inhibitors (21% vs. 25%) and chemotherapy (29% vs. 33%) after PD on study treatment was similar in cohort 1 and 2, respectively, but BRAF + MEK inhibitor therapy (43%) after PD could have prolonged OS in the BRAF-positive cohort compared to the BRAF-negative cohort.
Melanoma brain metastases are still associated with poor survival. Temozolomide has been studied in patients with melanoma brain metastases, but the median OS was short (3.5 months) in a large phase 2 study [Citation14]. Temozolomide, vincristine and lomustine are used in the treatment of gliomas and penetrate the central nervous system well [Citation15,Citation16]. Two BRAF-negative patients with baseline brain metastases had long survival (24 and +65.5 months) in our study. Surprisingly, OS and time to intracranial disease progression were not statistically significantly different in patients with or without baseline brain metastases. TOL-IFN might have intracerebral activity, but larger studies are needed to confirm our finding.
The use of combination chemotherapy in the first-line treatment of metastatic melanoma is not a standard option anymore [Citation17,Citation18], which makes further studies with TOL-IFN combination chemotherapy irrelevant in the first-line setting. Especially, patients with brain metastases have a very limited amount of treatment options and TOL-IFN could be a salvage option for them supplementing BRAF + MEK inhibitors, immune checkpoint inhibitors and stereotactic radiotherapy.
Conclusion
TOL-IFN chemoimmunotherapy showed similar efficacy in our study as dacarbazine-based chemotherapy in previous studies. Vemurafenib could be combined to TOL-IFN with acceptable toxicity after dose reductions and it improved ORR, PFS and OS of BRAF-positive patients. TOL-IFN might be considered as a salvage option for patients with melanoma brain metastases.
Author contributions
Kalle E. Mattila had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Hernberg was responsible for study concept and design. Mattila, Ramadan, Vihinen, Skyttä, Tiainen, Vuoristo, Tyynelä-Korhonen, Koivunen, Kohtamäki, Mäkelä and Hernberg were responsible for the acquisition of data. Mattila, Ramadan, Vihinen, Skyttä, Tiainen, Vuoristo, Tyynelä-Korhonen, Koivunen, Kohtamäki, Mäkelä and Hernberg were responsible for the analysis and interpretation of data. Mattila, Vihinen and Hernberg drafted the manuscript. Mattila, Ramadan, Vihinen, Skyttä, Tiainen, Vuoristo, Tyynelä-Korhonen, Koivunen, Kohtamäki, Mäkelä and Hernberg critically revised the manuscript for important intellectual content. Mattila was responsible for statistical analysis. Mattila and Hernberg obtained funding. Supervision was provided by Vihinen and Hernberg.
Supplemental Material
Download MS Word (28.5 KB)Disclosure statement
Kalle E. Mattila certifies that there are no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending).
Additional information
Funding
References
- Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47(14):2150–2157.
- Yang AS, Chapman PB. The history and future of chemotherapy for melanoma. Hematol Oncol Clin North Am. 2009;23(3):583–597. x. Epub 2009/05/26.
- Bajetta E, Del Vecchio M, Bernard-Marty C, et al. Metastatic melanoma: chemotherapy. Semin Oncol. 2002;29(5):427–445.
- Legha SS, Ring S, Eton O, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol. 1998;16(5):1752–1759.
- Pyrhonen S, Hahka-Kemppinen M, Muhonen T. A promising interferon plus four-drug chemotherapy regimen for metastatic melanoma. J Clin Oncol. 1992;10(12):1919–1926.
- Mattila K, Raanta P, Lahtela V, et al. Long-term survival of stage IV melanoma patients treated with BOLD combination chemotherapy and intermediate-dose subcutaneous interferon-alpha. Anticancer Res. 2018;38(11):6393–6397.
- Hodi FS, Chiarion-Sileni V, Gondalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492.
- Long GV, Flaherty KT, Stroyakovski D, et al. Dabrafenib plus trametinib versus dabreafenib monotherapy in patients with metastastic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–1639.
- Drummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiveing encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–1327.
- Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730.
- Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFv600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Common Terminology Criteria for Adverse Events (CTCAE)-EORTC. [cited 2019 Sep 19]. Available from: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the Treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101–2107.
- Van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350.
- Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high‐grade glioma. J Clin Oncol. 2010;28(30):4601–4610.
- Schadendorf D, Van Akkooi A, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984.
- Vuoristo MS, Hahka-Kemppinen M, Parvinen LM, et al. Randomized trial of dacarbazine versus bleomycin, vincristine, lomustine and dacarbazine (BOLD) chemotherapy combined with natural or recombinant interferon-alpha in patients with advanced melanoma. Melanoma Res. 2005;15(4):291–296.