Introduction
Glioblastoma multiforme (GBM), the commonest primary brain malignant tumor eludes a cure. The median overall survival (OS) continues to be a dismal 14 months with current standard of care which is concurrent Radiotherapy-Temozolomide (RT-TMZ) followed by adjuvant TMZ after maximal safe resection [Citation1].
One of the problems in achieving a gross total resection (GTR) in glioblastomas is its diffusely infiltrative nature. The tumor doubling time is estimated to be only 24 days emphasizing the aggressive growth of this tumor [Citation2,Citation3]. Radiobiology and tumor growth dynamics/kinetics of solid tumors suggest that every attempt should be made to start adjuvant therapy as early as possible to improve patient outcomes. RT has consistently shown to improve OS compared to best supportive care in GBM patients [Citation4,Citation5]. Clinical studies in head & neck, lung and breast cancer have consistently demonstrated higher local recurrence rates and poorer OS when adjuvant RT is delayed [Citation6–10]. Outcomes are poorer when RT is delayed even when RT is the definitive or primary treatment like cervical cancer [Citation11,Citation12]. In malignant gliomas however, published data is inconclusive. Some studies showed a negative impact on survival [Citation13–15], some no correlation [Citation16–18] and few reported improved survival with delay in initiating adjuvant RT [Citation19,Citation20].
Methods
Four hundred and twenty-five histopathologically confirmed supratentorial GBM patients treated between January 2008 and June 2017 with the standard protocol of maximal safe resection followed by concurrent TMZ and 60 Gy in six weeks of external RT, followed by six cycles of adjuvant TMZ were the subject of this retrospective analysis. The study was carried out at a single center tertiary care cancer hospital. All patients were treated on the same protocol during the entire study period. The hospitals’ institutional ethical committee approval was obtained for this study. Ineligibility criteria included patients less than 18 years, patients treated with only adjuvant RT without concurrent TMZ and patients who received less than four cycles of adjuvant TMZ after concurrent RT-TMZ schedule. Overall survival was measured in months from the date of surgery to the date of death or last follow-up. Patients who were alive at the time of last follow-up were classified as censored observations. Kaplan-Meier (KM) method was used to estimate survival rates. We classified patients into three groups based on surgery to RT time interval (SRTI): ≤10 days, 11–20 days and >20 days.
Statistical analysis was performed using SPSS version 20 software (SPSS Statistics). Log-Rank test was used to evaluate the significance of the association and Bonferroni correction was used for prognostic factors with more than two sub-categories. Univariate and multivariable analysis using Cox proportional hazards model with time on study time-scale were used to estimate Hazard ratio (HR) and 95% confidence intervals (CI) incorporating age, Karnofsky performance status (KPS), extent of surgery, recursive partitioning analysis (RPA) and SRTI, all as categorical variables. Age was categorized as two sub-groups: 18–54 and ≥55 years. KPS was categorized as ≥80, 60–79 and <60. RPA is a comprehensive scoring system for high grade gliomas introduced by the Radiation Therapy Oncology Group (RTOG) and is evaluated as Class III, IV or V based on age, KPS, neurological status and extent of resection. SRTI was also evaluated as a continuous variable in a linear functional form after testing for non-linearity. The primary end-point was OS and a P value less than 0.05 was considered as statistically significant.
Results
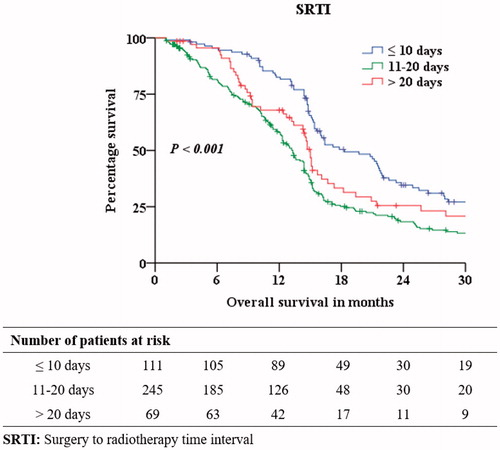
The median age was 54 years (Range: 18–82). There were 285 (67.1%) male and 140 (32.9%) female patients. The median SRTI was 15 days (Range: 5–124); SRTI groups ≤10 days, 11–20 days and >20 days had 111 (26.1%), 245 (57.6%) and 69 (16.3%) patients respectively. At a median follow-up of 45.6 months, the median OS was 14.7 months (). 391 (92.0%) patients completed the planned concurrent chemoradiation schedule. In our study, 112 (26.4%) patients were advised as fit to undergo RT by the neurosurgeon and were prepared to start the treatment in < =10 days. Amongst 313 (73.6%) patients who underwent RT after 10 days from date of surgery, 79 (25.2%) patients were advised to delay RT by neurosurgeons, 160 (51.2%) patients were fit to undergo RT but went home to meet family or work-related commitments, and came back to start RT, 74 (23.6%) patients had to delay RT owing to financial issues.
Age, KPS, extent of surgical resection, RPA and SRTI were all significant independent prognostic factors for survival. describes patient distribution across the SRTI groups. Sex and technique of RT did not affect survival. The 2-, 3- & 5-year OS was 23.9%, 13.3% and 6.9% respectively. Patients in SRTI ≤ 10 days had a 2-, 3- and 5-year survival of 34.6%, 20.0% and 14.3% respectively. Patients in SRTI 11–20 days had a 2-, 3- and 5-year survival of 18.3%, 9.8% and 3.4% respectively. Remaining 65 patients in SRTI ≥ 20 days had a 2-, 3- and 5-year survival of 25.5%, 13.9% and 6.9%. The median OS was 18.3, 13.3 and 15.0 months in SRTI groups ≤10 days, 11–20 days and >20 days respectively with chi-square value χ2(2) of 23.84 (p < .001). In log rank test, there was a statistically significant difference in survival distribution for STRI ≤10 days vs 11–20 days categories with chi-square value χ2(1) of 23.38 (p < .001), and between ≤10 days and >20 days with chi-square value χ2(1) of 5.76 (p = .016), but not between 11–20 days and >20 days with chi-square value χ2(1) of 2.83 (p = .093).
Table 1. Sample distribution of GBM patients based on surgery to radiotherapy time-interval.
In unadjusted and adjusted Cox regression model, SRTI as a continuous variable showed a 1.4 times increased risk of mortality with every 10 days’ delay in starting RT-TMZ following surgery (HR = 1.01, 95% CI, 1.00–1.03). When SRTI was analyzed as a categorical variable, the unadjusted HRs in SRTI 11–20 days and SRTI ≥ 20 days were 1.87 (95% CI, 1.45–2.41) and 1.44 (95% CI, 1.02–2.04) respectively when compared to patients in SRTI ≤ 10 days. The corresponding HRs when adjusted for age, KPS and surgery were 1.92 (95% CI, 1.48–2.41) and 1.57 (95% CI, 1.11–2.23) respectively. The corresponding HRs in multivariable analysis of OS when adjusted for RPA were 1.85 (95% CI, 1.43–2.39) and 1.43 (95% CI, 1.01–2.02).
Discussion
There are varied reasons for delay in initiating adjuvant RT after definitive treatment in cancer management. Some of them are long waiting times due to limited resources, lack of access to local RT facilities, machine overload, machine breakdown time, financial constraints, delay in wound healing and waiting for histopathology & molecular markers. Most of these factors are relevant to but not restricted to low income and middle-income countries. Patient should also be fit enough to undergo and complete the adjuvant treatment as overall RT treatment duration has also been implicated as a negative prognostic factor especially in cancer of the cervix [Citation21].
Studies in breast, head and neck and non-small cell lung cancer hence seem to confirm the hypothesis of presumed deleterious effect of delayed adjuvant RT on tumor control. In the context of high-grade gliomas, Blumenthal et al [Citation19] in one of the largest series to date analyzed 2800 patients from 16 Radiation Therapy Oncology Group (RTOG) studies and surprisingly reported a modest improvement in OS with delay of RT upto and beyond 4 weeks after surgery. On multivariate analysis, they found lower RPA class and time interval greater than 4 weeks as significant predictors of outcome. Noel et al [Citation16] and Lai et al [Citation18] did not find any statistically significant effect of timing of RT on OS in their patient cohort.
Three retrospective studies reported decreased survival in GBM patients when there was a delay in starting adjuvant RT. Do et al [Citation13] suggested that the time-interval between initial presentation and RT was detrimental to OS rather than the time-interval between surgery and radiation. They estimated a 2% increase in risk of death for each day delay in radiation. Irwin et al [Citation14] study on 172 patients reported that an 8-week waiting period reduced the median OS by 11 weeks compared with a 2-week waiting period for RT. Glinski et al [Citation15] reported a significantly worse OS when the delay in starting RT was more than 37 days in their experience with 308 patients of high grade gliomas. There are other studies giving indirect evidence supporting the hypothesis of poorer outcome with delay in initiating adjuvant RT. One such study by Chinot et al [Citation22] also emphasizes the importance of starting adjuvant radiation early in GBM management. They used a neoadjuvant dense dose TMZ schedule of 2 months before the standard concurrent RT-TMZ. This treatment approach was inferior to standard concurrent RT-TMZ. Our study gives additional evidence for the detrimental effect of delaying adjuvant chemoradiation.
The data is therefore conflicting and inconclusive regarding the impact of adjuvant RT-TMZ timing on OS in GBM patients. The results from all the above studies must be interpreted with certain caveats;
Most of the published literature in gliomas deals with anaplastic gliomas and glioblastomas together. These are two distinct biological entities with different behavior and clinical outcomes and hence need to be considered separately. Our study considered only histologically proven GBM patients.
The mean time to starting adjuvant RT after surgery was very variable (range 14 days to 60 days). Most studies used the median length of delay as the cut off. A few studies used percentiles to categorize the waiting time while a few other studies treated waiting time to RT as a continuous variable. The median time from surgery to RT-TMZ in our study was 15 days. Majority of the patients started RT-TMZ within 4 weeks.
Most of the studies discussed above were in the pre-TMZ era and hence may not be relevant in the present era of concurrent RT-TMZ for GBM. Noel et al [Citation16] is the only study where all patients received the modern protocol of concurrent and adjuvant TMZ uniformly. They used percentiles for modeling surgery to RT waiting time into categories. This may fail to detect effects of smaller magnitude. All the patients in our cohort received TMZ.
The reasons for delay in starting concurrent RT-TMZ in our patients was varied. Most of our patients had traveled away from home to undergo the treatment and had to make arrangements for an extended stay near the hospital. The approval from neurosurgeon after evaluating wound healing and neurological fitness of the patient also delays radiation. Other reasons include social and financial constraints of the patient and caregiver. In our study, the technique of RT did not have any effect on the OS rate of glioblastoma patients. Chen et al, reported a similar result in a study that evaluated the effect of 3 D-CRT and IMRT on 1-year OS (89.6% vs. 75.8%, p = .795) and 1-year progression free survival (61.0% vs. 45.5%, p = .867) in 54 GBM patients. Study by Thibouw et al on the effect of 3 D-CRT vs IMRT in 220 glioblastoma patients reported reduced neurological toxicities but no significant difference in median OS (16.0 vs 13.4 months, p = .542).
The limitation of our study is its retrospective nature as are all other studies discussed too. It is unlikely that a prospective randomized study can ever be conducted due to ethical concerns of deliberately delaying an adjuvant treatment. The main strength of this study is the large number of patients in our cohort treated on a uniform protocol with adequate follow-up at a single center. We believe our study strengthens the hypothesis that adjuvant RT-TMZ should be started as early as possible after surgery, preferably within 10 days to improve OS in GBM patients.
Conclusion
Delay in starting adjuvant RT-TMZ beyond 10 days appears to be detrimental in GBM patients in our study.
Disclosure statement
All the authors declare no conflict of interest.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
- Burnet NG, Jena R, Jefferies SJ, et al. Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol (R Coll Radiol). 2006;18(2):93–103.
- Taghian A, Ramsay J, Allalunis-Turner J, et al. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1993;25(2):243–249.
- Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant gliomas after surgery. N Engl J Med. 1980;303(23):1323–1329.
- Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535.
- Froud PJ, Mates D, Jackson JS, et al. Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys. 2000;46(2):363–372.
- Vujovic O, Perera F, Dar AR, et al. Does delay in breast irradiation following conservative breast surgery in node-negative breast cancer patients have an impact on risk of recurrence? Int J Radiat Oncol Biol Phys. 1998;40(4):869–874.
- Choi N, Baumann M, Flentjie M, et al. Predictive factors in radiotherapy for non-small cell lung cancer: present status. Lung Cancer. 2001;31(1):43–56.
- Ampil FL, Buechter KJ, Bairnsfather LE, et al. Timing and dosage of postoperative radiotherapy for squamous cell carcinoma of the upper aerodigestive tract. J Oral Maxillofac Surg. 1993;51(11):1194–1197.
- Bastit L, Blot E, Debourdeau P, et al. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2001;49(1):139–146.
- Perri T, Issakov G, Ben-Baruch G, et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24(7):1326–1332.
- E Choan, Dahrouge S, Samant R, Mirzaei A, et al. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int J Radiat Oncol Biol Phys. 2005;61(4):1071–1077.
- Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57(2):131–136.
- Irwin C, Hunn M, Purdie G, et al. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007;85(3):339–343.
- Gliński B, Urbański J, Hetnał M, et al. Prognostic value of the interval from surgery to initiation of radiation therapy in correlation with some histo-clinical parameters in patients with malignant supratentorial gliomas. Contemp Oncol (Pozn). 2012;16(1):34–37.
- Noel G, Huchet A, Feuvret L, et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. 2012;109(1):167–175.
- Lutterbach J, Weigel P, Guttenberger R, et al. Accelerated hyper-fractionated radiotherapy in 149 patients with glioblastoma multiforme. Radiother Oncol. 1999;53(1):49–52.
- Lai R, Hershman DL, Doan T, et al. The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro Oncol. 2010;12(2):190–198.
- Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739.
- Wehming FM, Wiese B, Nakamura M, et al. Malignant glioma grade 3 and 4: how relevant is timing of radiotherapy? Clin Neurol Neurosurg. 2012;114(6):617–621.
- Fyles A, Keane TJ, Barton M, et al. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol. 1992;25(4):273–279.
- Chinot OL, Barrié M, Fuentes S, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. JCO. 2007;25(12):1470–1475.