Abstract
Background: Fluoropyrimidines are mainstay chemotherapeutics in the treatment of gastrointestinal cancers and are also used to treat breast cancer and head and neck cancers. However, 5-flourouracil (5-FU) and capecitabine may induce cardiotoxicity that mostly presents as acute coronary syndromes. We compared the incidence of cardiotoxicity induced by 5-FU and capecitabine in patients with colorectal cancer and sought to identify risk markers for cardiotoxicity.
Methods: We reviewed all consecutive patients with colorectal cancer who received 5-FU or capecitabine at one institution in the neoadjuvant (2007–2016), adjuvant (2000–2016) or metastatic setting (2007–2016).
Results: Totally, 995 patients received 5-FU and 1241 received capecitabine. The incidence of cardiotoxicity induced by 5-FU was 5.2% [95% confidence interval (CI): 3.8–6.6%] and 4.1% (95% CI: 3.0–5.2%) induced by capecitabine (p = .21). The most common events were angina without ischemia (5-FU: 1.6%, capecitabine: 1.3%, p = .53), angina with ischemia on ECG (5-FU: 0.9%, capecitabine: 0.8%, p = .53), unspecified chest pain (5-FU: 0.9%, capecitabine: 0.6%, p = .34), ST-elevation myocardial infarction (5-FU: 0.5%; capecitabine: 0.4%, p = .76) and non-ST-elevation myocardial infarction (5-FU: 0.7%, capecitabine: 0.5%, p = .50). Cardiac arrest or sudden death occurred in 0.5 and 0.4%, respectively (p = 1). No risk markers for cardiotoxicity induced by 5-FU were identified. In the capecitabine group, ischemic heart disease was a risk marker (odds ratio: 2.9, 95% CI: 1.2–7.0, p = .016).
Conclusions: Five percent of patients treated with 5-FU developed cardiotoxicity and 4% treated with capecitabine. Ischemic heart disease was a risk marker for cardiotoxicity induced by capecitabine.
Background
5-Fluorouracil (5-FU) and its oral pro-drug, capecitabine, are key chemotherapeutic agents in the treatment of gastrointestinal cancers [Citation1–8] and are also used to treat metastatic breast cancer and head and neck cancer [Citation9–11]. Acute cardiotoxicity is a potentially severe adverse event associated with 5-FU and capecitabine treatment [Citation12–15].
Cardiotoxicity typically presents as chest pain with or without electrocardiographic signs of myocardial ischemia during or shortly after chemotherapy, but also myocardial infarction, cardiac arrhythmias, left ventricular dysfunction and sudden death can occur [Citation12–15]. The incidence of cardiotoxicity induced by 5-FU ranges from 0.55 to 19.9% in published studies, while cardiotoxicity induced by capecitabine has been reported to be 3–35% [Citation12]. Prior studies directly comparing the incidence of cardiotoxicity induced by capecitabine with continuous and bolus 5-FU infusion schedules have reported divergent results [Citation16–18]. One study reported lower incidence of cardiotoxicity for short 5-FU infusion compared to capecitabine and continuous 5-FU infusion schedules [Citation17], while another study reported comparable incidences for bolus 5-FU infusion and capecitabine [Citation18].
Although cardiotoxicity induced by 5-FU was first described in 1969 [Citation19], the pathophysiology remains unclear [Citation20] and risk markers for cardiotoxicity are largely unknown [Citation12–14]. Cardiac comorbidities and continuous infusion schedules have been associated with an increased risk of cardiotoxicity [Citation16,Citation17,Citation21–24], but there are divergent results [Citation21,Citation25–29], and many studies lack statistical power.
Consequently, more research on risk markers is needed in large cohorts to improve risk stratification and guide treatment selection. We compared the incidence and patterns of cardiotoxicity in patients with colorectal cancer treated with 5-FU or capecitabine and sought to identify risk markers.
Material and methods
Selection of patients
We included patients with colorectal cancer who received 5-FU- or capecitabine-based chemotherapy in the adjuvant (2000–2016), neoadjuvant (2007–2016) or the metastatic setting (2007–2016) at the Department of Oncology, Herlev-Gentofte University Hospital, Denmark. Medical records for all consecutive patients with colorectal cancer referred to the department during these periods were reviewed and evaluated for eligibility. Inclusion criteria were a diagnosis of colorectal cancer and first-time treatment with 5-FU or capecitabine. All patients receiving at least one dose were included. Patients with missing medical records were excluded (). 5-FU were administered according to the modified de Gramont schedule (bolus plus 46-h continuous infusion), while capecitabine were given daily for 7 days, 14 days or continuously (Supplementary Table A.1). Doses and treatment regimens are described in Supplementary Table A.1.
Data collection
The following data were collected: age, sex, cardiovascular comorbidities (ischemic heart disease, heart failure or reduced ejection fraction, cardiac arrhythmias, valvular heart diseases, cardiomyopathies, stroke or transient cerebral ischemia and peripheral atherosclerotic disease), risk factors for coronary heart disease (hypertension, hypercholesterolemia, smoking status, diabetes), hemoglobin and creatinine, height, weight, antineoplastic treatment, duration of treatment and starting dose (Supplementary Table A.2). Approval was obtained from the Danish Health Authority and the Danish Data Protection Agency.
Endpoints and evaluation of cardiotoxicity
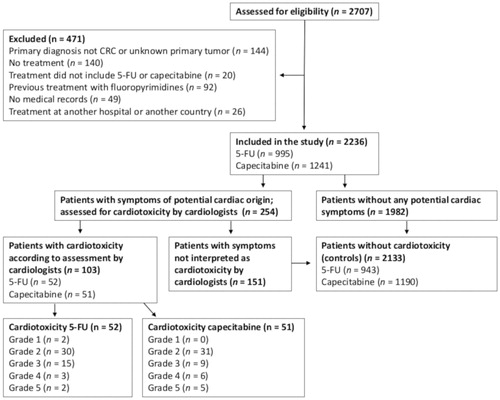
The primary endpoint was cardiotoxicity defined as symptoms or events of likely cardiac origin that presented during or ≤7 days after the last dose of 5-FU or capecitabine and were absent before treatment start. Unexpected sudden death and cardiac arrest within this period were included as being of cardiac origin if no other cause was identified. As a routine, all patients were asked about cardiac symptoms before start of chemotherapy and before each cycle. ECGs were routinely performed before treatment initiation. All patients were assessed for symptoms of possible cardiac origin (chest pain, dyspnea, palpitations etc.) by one of the authors (AD-P, CL, KV or DN). Patients with symptoms of possible cardiac origin were reviewed by an endpoint committee consisting of four experienced cardiologists to decide whether they had cardiotoxicity or not (MS, MVN, TK, JMA) (). Each case was reviewed by two members of the committee independently. All disagreements (n = 15) were solved by discussion until consensus was reached. The committee had access to medical records including details about treatment, comorbidities and cardiac examinations. If another diagnosis was more likely or the cardiologists considered the symptoms not likely to be of cardiac origin, the patients were interpreted as not having cardiotoxicity (). Patients with cardiotoxicity were graded according to Common Terminology Criteria for Adverse Events, version 4.03.
Statistics
The Mann–Whitney U-test was used to compare differences in age between the two groups. Fisher's exact test and the χ2 test were used to compare differences in numeric variables, and the χ2 test for trend was used to compare ordinal variables between groups. Possible risk markers for cardiotoxicity were tested using univariate binomial logistic regression. Sensitivity analyses with imputation of worst- and best-case scenarios were performed for variables significant in univariate analyses to test the robustness of the data. A multivariate binomial logistic regression model was fitted for variables with p ≤ .05 in univariate analyses. A variance inflation factor was calculated to assess collinearity among variables in the multivariate model. C-statistics were performed for variables that were significant in multivariate analyses. p values < .05 (two-sided) were regarded significant. IBM SPSS software V.21 was used for all analyses.
Results
Study population
Of the 2707 patients screened, 2236 were included (). Treatment included 5-FU in 995 patients (44.5%) and capecitabine in 1241 patients (55.5%). Clinical characteristics are given in . Patients in the capecitabine group (median age 70 years, range 22–93) were older than patients in the 5-FU group (median age 65 years, range 21–85) (p < .001), received more often bevacizumab (5-FU group: 2.1%; capecitabine group: 8.6%, p < .001) and were less often treated with epidermal growth factor receptor (EGFR) inhibitor (p < .001). Furthermore, more patients in the capecitabine group had rectal cancer (p < .001), received neoadjuvant treatment (p < .001), had performance status > 1 (p = .001), had hypertension (p = .001) and had a low estimated glomerular filtration rate (p = .020).
Table 1. Patient characteristics.
Cardiotoxicity
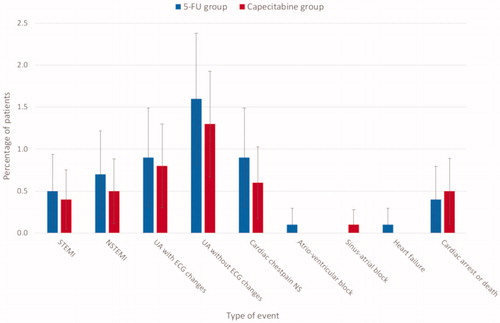
In the group of patients receiving 5-FU, the incidence of cardiotoxicity was 5.2% [N = 52, 95% confidence interval (CI): 3.8–6.6%] compared to 4.1% (N = 51, 95% CI: 3.0–5.2%) in the capecitabine group (p = .21). No significant differences in the type of events were observed between the two groups (). The most frequent cardiac event was acute coronary syndromes (5-FU: 4.6%; capecitabine: 3.6%) (Supplementary Table B.1). According to the criteria for acute coronary syndromes, ST-elevation myocardial infarction occurred in 0.5% treated with 5-FU and 0.4% treated with capecitabine (p = .76) and non-ST myocardial infarction occurred in 0.7 and 0.5% (p = .50), respectively. Unstable angina with ischemia on ECG was seen in 0.9 and 0.8% (p = .53) and angina without ischemia on ECG occurred in 1.6 and 1.3%, respectively (p = .53). Chest pain without assessment of ECG and troponin occurred in 0.9% (5-FU) and 0.6% (capecitabine) (p = .34). Heart failure (0.1%) and atrioventricular block (0.1%) each occurred in one patient treated with 5-FU, and sinus arrest was observed in one patient receiving capecitabine (0.08%). Four sudden deaths or cardiac arrests (0.4%) were identified in the 5-FU group and six (0.5%) in the capecitabine group (p = 1.00) (Supplementary Table B.2). Among 24 patients (5-FU: 13; capecitabine: 11) with cardiotoxicity that underwent coronary angiography, only three had significant stenosis (13%).
Figure 2. The incidence of cardiac events in the 5-FU and capecitabine groups. The figure shows the percentage of cardiac events for patients with colorectal cancer treated with 5-FU-based or capecitabine-based chemotherapy. The error bars show 95% confidence limits. There were no significant differences in incidence of any type of event between the two groups. NSTEMI: non-ST-elevation myocardial infarction; NS: not specified; STEMI: ST-elevation myocardial infarction; UA: unstable angina.
In the 5-FU group, 2 grade 1 toxicities (0.2%), 30 grade 2 toxicities (3.0%), 15 grade 3 toxicities (1.5%), 3 grade 4 toxicities (0.3%) and 2 grade 5 toxicities (0.2%) were observed (, Supplementary Tables B.1 and B.2). In the capecitabine group, 0 grade 1 toxicities, 31 grade 2 toxicities (2.5%), 9 grade 3 toxicities (0.7%), 6 grade 4 toxicities (0.5%) and 5 grade 5 toxicities (0.4%) were found. There was no overall difference in the grade of cardiotoxicities between the 5-FU group and the capecitabine group (p = .41) and the incidence of grade 3–4 cardiotoxicities was comparable in the two groups (5-FU: 1.8%; capecitabine: 1.2%, p = .16).
Most cardiotoxic events occurred in the first cycle (5-FU: 67%; capecitabine: 59%), followed by the second (14 versus 14%), third (6 versus 14%) and fourth (4 versus 4%) cycles. Onset of cardiotoxicity in later cycles was seen in 10% of the 5-FU patients and 10% of capecitabine patients (p = .75).
In patients treated with capecitabine, the incidence of cardiotoxicity was higher among patients with ischemic heart disease than in those without (11.8 versus 3.7%, p = .005). There was no significant difference in the incidence of cardiotoxicity between patients with or without ischemic heart disease in the 5-FU group (9.4 versus 5.0%, p = .19).
In patients experiencing cardiotoxicity, treatment was stopped in 34 patients (65%) receiving 5-FU and in 35 patients (73%) receiving capecitabine, while 18 patients (35%) were retreated in the 5-FU group and 14 (27%) in the capecitabine group. After dose reduction and/or initiation of cardiac therapy with isorbide mononitrate or a calcium antagonist, 44–75% of the patients had recurrent symptoms at retreatment ().
Table 2. The effect of dose reduction and/or cardiac therapy in patients retreated with 5-FU or capecitabine.
Risk markers for cardiotoxicity
In the 5-FU group, no risk markers were identified in univariate logistic regression analyses (). In the capecitabine group, univariate logistic regression analyses revealed significant results for ischemic heart disease (OR 3.49, 95% CI 1.57–7.76, p = .002) and hypercholesterolemia (OR 2.06, 95% CI 1.12–3.79, p = .020). Data on ischemic heart disease was missing in 0.3% (N = 4) and data on hypercholesterolemia in 1.0% (N = 13) in the capecitabine group. After sensitivity analyses with best- and worst-case scenarios, both ischemic heart disease [p = .002 (best-case), p = .003 (worst-case)] and hypercholesterolemia [p = .036 (best-case), p = .004 (worst-case)] remained significant. Only ischemic heart disease was significant in the multivariate model (). Patients with ischemic heart disease were 2.9 (OR) (95% CI: 1.2–7.0, p = .016) times more likely to develop cardiotoxicity induced by capecitabine than patients without ischemic heart disease.
Table 3. Univariate and multivariate logistic regression models of risk markers for cardiotoxicity.
Prediction of cardiotoxicity
Using C-statistics, we tested the ability of ischemic heart disease to discriminate between patients who developed cardiotoxicity induced by capecitabine and those who did not. We found that ischemic heart disease (C = 0.55, 95% CI: 0.47–0.64, p = .20) was a poor predictor of cardiotoxicity.
Discussion
We found comparable incidences and no differences in the patterns of cardiotoxicity induced by 5-FU and capecitabine in patients with colorectal cancer. Angina without ECG changes and elevated troponin were the most common event followed by angina with ECG changes. More severe events such as ST-elevation myocardial infarction and non-ST-elevation myocardial infarction were less frequent, albeit cardiac arrest or sudden death was seen in 1 per 250 patients. Analyses for risk markers suggested that ischemic heart disease is associated with increased risk of cardiotoxicity in patients treated with capecitabine. No risk markers for cardiotoxicity induced by 5-FU were identified.
Overall, the patterns and incidences of cardiac events in this study agree with findings in previous studies [Citation12], although some studies have reported higher incidences of cardiac arrhythmias [Citation21,Citation30,Citation31]. The observed incidence of sudden death or cardiac arrest in 1 per 250 treated patients is high but in line with previous studies. The cause of sudden death was unclear in most patients because autopsy was only available in one case. Hence, we might have overestimated the incidence of sudden death caused by 5-FU and capecitabine, since some of the patients could have died from a pulmonary embolus or other complications. However, the continuous reporting of a temporal association between fluoropyrimidine treatment and sudden death or cardiac arrest suggests a causal relation between the treatment and serious adverse outcomes. Furthermore, in three patients that were successfully resuscitated, no other reasons for cardiac arrest were identified and coronary angiography revealed no stenosis.
Only 24 patients underwent coronary angiography in this retrospective study from 2000 to 2016. It may be explained by the fact that the treating clinicians meant the symptoms and rise in troponins or changes in ECG were induced by 5-FU or capecitabine treatment. However, coronary angiography only revealed stenosis in three of the 24 patients, which supports the theory of vasospasm as a potential mechanism for cardiotoxicity.
Our study suggests that ischemic heart disease is a risk marker for capecitabine cardiotoxicity, but we could not demonstrate the same association for 5-FU. Since the prevalence of ischemic heart disease was similar in the two groups, differences in prevalence of ischemic heart disease cannot explain the findings. Also, it is unlikely that the differences between the groups with regard to median age and prevalence of hypertension influenced the findings, because these variables were not associated with increased risk of cardiotoxicity. Possible explanations for the findings could be (1) that risk markers for cardiotoxicity for the two treatments may be different or (2) a type II statistical error due to an insufficient sample size in the 5-FU group.
Significant associations between ischemic heart disease and cardiotoxicity have been reported in one study of capecitabine [Citation22] and in two studies of 5-FU [Citation23,Citation24], while one study of capecitabine and one of 5-FU found no association [Citation26,Citation27]. Likewise, preexisting cardiac disease of any type has been associated with increased risk of cardiotoxicity in two studies of capecitabine [Citation22,Citation32] and three studies of 5-FU [Citation23,Citation24,Citation29], but some studies found no association [Citation21,Citation25–27,Citation33].
Other potential risk markers of cardiotoxicity have been less consistently studied. In this study and in a prior study of cardiotoxicity induced by capecitabine [Citation32], hypercholesterolemia was significant in univariate analyses. However, in this study hypercholesterolemia was not significant in multivariate analyses, and a prospective study in which cholesterol and triglyceride levels were measured could not demonstrate an association [Citation17]. Smoking has been associated with cardiotoxicity in two studies [Citation17,Citation32], while we found no association with smoking status in this study [Citation12,Citation19,Citation22,Citation25,Citation31].
Furthermore, the combination of 5-FU or capecitabine with bevacizumab results in higher incidences of cardiotoxicity [Citation30,Citation31]; however, we were not able to demonstrate an association between bevacizumab and cardiotoxicity, which might be due to the low number of patients receiving bevacizumab in our study.
The effect of dose reduction and/or antianginal therapy on cardiac symptoms during retreatment was modest to poor in this study. Previous studies have reported slightly better outcomes [Citation16,Citation17], but the evidence is limited to case series. The impact of dose reductions on cancer recurrence or progression is not well documented. A retrospective study of adjuvant chemotherapy for stage III colon cancer reported no association between relative dose intensity and recurrence-free or overall survival [Citation34], while another study found better 5-year overall survival for relative dose intensities >70% compared to relative dose intensities <70% [Citation35]. In the metastatic setting, two retrospective studies found no effect of dose reduction or low dose intensities on overall survival and time to treatment failure [Citation36,Citation37]. Thus, the scientific evidence to guide the clinical decision regarding whether or not to retreat with these drugs is sparse, but the minor risk of severe cardiotoxicity should be weighed against the expected treatment benefit.
If retreatment with 5-FU or capecitabine is decided, bolus 5-FU infusion may be an alternative. In a case-series of 10 patients with 5-FU or capecitabine cardiotoxicity, no further cardiac symptoms were observed during retreatment with bolus 5-FU infusion and oxaliplatin [Citation38]. However, as mentioned in the introduction, it is unclear whether the incidence of cardiotoxicity from bolus 5-FU is lower than for continuous 5-FU infusion schedules and capecitabine. One study reported lower incidences of cardiotoxicity for short 5-FU infusion compared to capecitabine and continuous 5-FU infusion [Citation17], while comparable incidences for bolus 5-FU infusion and capecitabine was found in another study [Citation18].
Alternative treatment options in patients with colorectal cancer include UFT, S-1 or raltitrexed. In UFT and S-1, the 5-FU prodrug, tegafur, are combined with a dihydropyrimidine dehydrogenase (DPD) inhibitor that reduces the degradation of 5-FU to fluorobetaalanine [Citation39]. Since fluorobetaalanine may be involved in the cardiotoxic effects from fluoropyrimidines [Citation40], DPD inhibition should result in lower incidences of cardiotoxicity. For UFT, lower incidences of cardiotoxicity (1%) have been reported [Citation41], but there are few published data regarding the cardiotoxicity from S-1. Yet, a case series of seven patients whom were successfully treated with S-1 after cardiotoxicity induced by capecitabine has been published [Citation42]. The thymidylate synthase inhibitor, raltitrexed, is an alternative in patients with advanced colorectal cancer. In 42 patients with cardiotoxicity induced by 5-FU or capecitabine, no further cardiac events were reported during treatment with raltitrexed [Citation43]. However, raltitrexed has been associated with a high mortality rate (2–6%) [Citation44].
There are some limitations to our study. It is retrospective, which may have resulted in an underestimation of the incidence of cardiotoxicity due to incomplete data and the overlooking of patients with mild symptoms. Furthermore, the definition of cardiotoxicity is subjective, and there is no objective test to diagnose fluoropyrimidine cardiotoxicity. The diagnosis is based on symptom interpretation, the temporal association between symptoms and drug administration and lack of other more likely causes. Thus, it is a challenge to detect and rule out fluoropyrimidine cardiotoxicity, and the diagnosis is therefore hampered by subjective elements.
We performed several univariate logistic regression analyses to identify risk markers, which increases the risk of type I statistical errors. Lastly, 5-FU cardiotoxicity is a rare side effect. Hence the number of events is often low, which compromises the power of the statistical analyses. Our study is among the larger studies of fluoropyrimidine cardiotoxicity. Still, power can be a problem because both the event and some of the conditions evaluated as risk markers are rare.
In conclusion, the incidence of cardiotoxicity induced by 5-FU and by capecitabine are similar 5 and 4%, respectively. Ischemic heart disease may be a risk marker for capecitabine cardiotoxicity. No risk markers for 5-FU cardiotoxicity were identified. Future studies of risk markers for 5-FU and capecitabine cardiotoxicity should be large-scale, international, prospective studies with careful registration of comorbidities and cardiotoxic symptoms. New approaches to the identification of patients at risk of cardiotoxicity are highly warranted, and future studies should evaluate whether inter-individual genetic variability influences the risk of 5-FU- and capecitabine-induced cardiotoxicity.
Ethics approval and consent to participate: This study was performed according to the Declaration of Helsinki and approved by the Danish Health Authority and the Danish Data Protection Agency.
Author contributions
AD-P contributed to the conception and design of the study, data collection, data processing, statistics, writing of the paper and incorporation of input from the other authors. MV-N contributed to the conception and design of the study, reviewed cases with suspected cardiotoxicity and reviewed the manuscript. MS contributed to the conception and design of the study, reviewed cases with suspected cardiotoxicity, was involved in the interpretation of study results and statistics and reviewed the manuscript. KKV contributed to the conception and design of the study, data collection and review of the manuscript. CML contributed to data collection and review of the manuscript. TK and JMA were involved in review of cases with suspected cardiotoxicity and review of the manuscript. DLN contributed to the conception and design of the study, data collection, data interpretation, input to the manuscript and review of the manuscript.
Supplemental Material
Download Zip (96.9 KB)Acknowledgments
The authors would like to thank Terkel Christiansen and Andreas Bartholdy for help with data extraction from medical records and Professor Stig Egil Bojesen and the Department of Clinical Biochemistry at Herlev and Gentofte University Hospital for providing data on creatinine and hemoglobin.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. on behalf of the ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Van Cutsem E, Cervantes A, Nordlinger B, et al. on behalf of the ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 (Suppl 3):iii1–9.
- Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 (Suppl 3):iii10–20.
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl_5):v38–v49.
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl_5):v50–v57.
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl_5):v28–v37.
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 (Suppl 5):v56–68.
- Blum JL, Barrios CH, Feldman N, et al. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2012;136(3):777–788.
- O'Shaughnessy JA, Kaufmann M, Siedentopf F, et al. Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer. Oncologist. 2012;17(4):476–484.
- Gregoire V, Lefebvre JL, Licitra L, et al. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Med Oncol. 2010;21 (Suppl 5):v184–6.
- Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–984.
- Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191–202.
- Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140.
- Sorrentino MF, Kim J, Foderaro AE, et al. 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J. 2012;19(5):453–458.
- Jensen SA, Sorensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58(4):487–493.
- Kosmas C, Kallistratos MS, Kopterides P, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2007;134(1):75–82.
- Van Cutsem E, Hoff PM, Blum JL, et al. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol. 2002;13(3):484–485.
- Gaveau T, Banzet P, Marneffe H, et al. Cardiovascular disorders in the course of antimitotic infusions at high doses. 30 clinical cases. Anesth Analg. 1969;26(3):311–327.
- Polk A, Vistisen K, Vaage-Nilsen M, et al. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15(1):47.
- Khan MA, Masood N, Husain N, et al. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) and 5-FU based chemotherapy regimens in Pakistani adult cancer patients at Shaukat Khanum Memorial Cancer Hospital & Research Center. J Pak Med Assoc. 2012;62(5):430–434.
- Koca D, Salman T, Unek I, et al. Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy. 2011;57 (5):381–387.
- Labianca R, Beretta G, Clerici M, et al. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori. 1982;68(6):505–510.
- Meyer CC, Calis KA, Burke LB, et al. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy. 1997;17(4):729–736.
- Jensen SA, Hasbak P, Mortensen J, et al. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. JCO. 2010;28(36):5280–5286.
- Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont's regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. 2005;35(5):265–270.
- Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer. 2005;41(11):1542–1546.
- Wacker A, Lersch C, Scherpinski U, et al. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology. 2003;65(2):108–112.
- Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. JCO. 1989;7(4):509–514.
- Abdel-Rahman O. 5-Fluorouracil-related cardiotoxicity; findings from five randomized studies of 5-fluorouracil-based regimens in metastatic colorectal cancer. Clin Colorectal Cancer. 2019;18(1):58–63.
- Kwakman JJ, Simkens LH, Mol L, et al. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer. 2017;76:93–99.
- Polk A, Shahmarvand N, Vistisen K, et al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open. 2016;6(10):e012798.
- Lestuzzi C, Vaccher E, Talamini R, et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Med Oncol/ESMO. 2014;25(5):1059–1064.
- Smoragiewicz M, Javaheri KR, Yin Y, et al. Neutropenia and relative dose intensity on adjuvant FOLFOX chemotherapy are not associated with survival for resected colon cancer. J Gastrointest Cancer. 2014;45(4):460–465.
- Aspinall SL, Good CB, Zhao X, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer. 2015;15(1):62.
- Mochinaga S, Okahashi T, Koga S, et al. Effects of reduced dose intensity of modified FOLFOX6 in patients with metastatic or recurrent colorectal cancer. Oncol Res. 2011;19(10):511–518.
- Munker S, Gerken M, Fest P, et al. Chemotherapy for metastatic colon cancer: no effect on survival when the dose is reduced due to side effects. BMC Cancer. 2018;18(1):455.
- Chakrabarti S, Sara J, Lobo R, et al. Bolus 5-fluorouracil (5-FU) in combination with oxaliplatin is safe and well tolerated in patients who experienced coronary vasospasm with infusional 5-FU or capecitabine. Clin Colorectal Cancer. 2019;18:52–57.
- Deboever G, Hiltrop N, Cool M, et al. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin Colorectal Cancer. 2013;12(1):8–14.
- Lemaire L, Malet-Martino MC, de FM, et al. Cardiotoxicity of commercial 5-fluorouracil vials stems from the alkaline hydrolysis of this drug. Br J Cancer. 1992;66(1):119–127.
- Yamamoto J, Haruno A, Yoshimura Y, et al. Effect of coadministration of uracil on the toxicity of tegafur. J Pharm Sci. 1984;73(2):212–214.
- Kwakman JJM, Baars A, van Zweeden AA, et al. Case series of patients treated with the oral fluoropyrimidine S-1 after capecitabine-induced coronary artery vasospasm. Eur J Cancer. 2017;81:130–134.
- Ransom D, Wilson K, Fournier M, et al. Final results of Australasian Gastrointestinal Trials Group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 2014;25(1):117–121.
- Cunningham D, the "Tomudex" Colorectal Cancer Study Group, Zalcberg JR, Rath U, et al. Final results of a randomised trial comparing 'Tomudex' (raltitrexed) with 5-fluorouracil plus leucovorin in advanced colorectal cancer. “Tomudex” Colorectal Cancer Study Group. Ann Oncol. 1996;7(9):961–965.