Introduction
Although anti-programmed death (PD)-1 drugs have less adverse effects (AEs) than standard chemotherapy, they may cause distinctive immune-related AEs (IRAEs) caused by immune system stimulation in normal tissue [Citation1]. The incidence of immune checkpoint inhibitor (ICI) -associated myocarditis is believed to be low [Citation2]. Myocarditis is more common in those patients administered combination ICI therapies in comparison to patients who receive single-agent treatments [Citation3,Citation4] and usually occurs early in the course of the treatment [Citation4]. Symptomatic ICI-associated myocarditis has been reported to have 50% mortality; according to the published results, the likelihood of death is more pronounced among patients receiving ICI combination therapy vs. ICI monotherapy [Citation5].
Case report
A 75-year-old woman with a medical history of hypertension and macular pucker had been followed-up for a pT4bN0, Breslow 5 mm nodular melanoma (ulceration status: positive, mitotic count 4/mm2) detected in January 2014. No adjuvant therapies were primarily prescribed for the stage IIC disease. As a part of the follow-up, she underwent whole-body computed tomography (CT) imaging in summer 2018. New lung nodules were detected and the largest, 10 mm in size, was eventually surgically removed with thoracoscopy confirming the diagnosis of metastatic melanoma. Next generation sequencing was conducted and a BRAF V600E mutation was detected. After signing a written informed consent form, she was enrolled in a clinical study investigating nivolumab and placebo vs. nivolumab and the LAG3-inhibitor relatlimab.
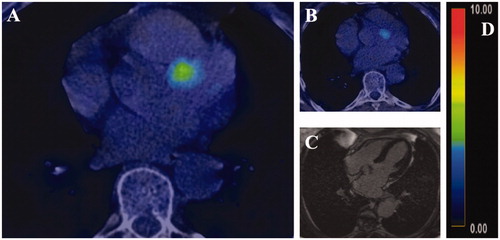
The troponin T concentration was 15 ng/L at the baseline (reference value < 15 ng/L) (day 0 (D0)) and remained low at the beginning of the follow-up (). After the third cycle, CT indicated that the pulmonary nodules had shrunk. After the fourth cycle (D127), the patient reported mild (grade 1) symptoms: fatigue, peripheral edema and pruritus. At this time point, the troponin T concentration was mildly elevated (17 ng/L). The patient was referred to a cardiologist who detected no abnormal findings in the cardiopulmonary status or in the 12-lead electrocardiogram. The transthoracic echocardiography finding was normal with a left ventricular ejection fraction > 60% and the pro-brain natriuretic peptide (proBNP) 366 (reference value < 738 ng/L). The study medication was discontinued because of evolving multiple, yet mild adversary effects in agreement with the patient’s preference. Due to the fact that troponin T values were only mildly elevated and the good overall wellbeing of the patient, glucocorticoid therapy was not initiated. When the patient developed a persistent case of hypoalbuminemia, and was suspected of having intestinal malabsorption, a moderate-dose oral glucocorticoid therapy (prednisolone, 40 mg once a day) was initiated on D175. After a week’s use (D175-183), the glucocorticoid therapy was reduced from the daily dose of 40 mg–20 mg. All of the other symptoms including the hypoalbuminemia resolved, but the troponin T concentration was increasing despite the glucocorticoid therapy. Therefore, the patient was referred to cardiac FDG-PET-CT (D216) that revealed diffuse activity in the left ventricle and a focal area with SUVmax of 5.4 at the base of the septum (shown in ) having a resemblance to myocarditis. Nonetheless, cardiac magnetic resonance imaging (cMRI) (D218) with routine sequences, including T1 and T2 mapping sequences, showed no focal changes (). As the troponin T level slowly declined, the glucocorticoid dose was reduced first to 10 mg and then to 5 mg on days 202 and 267, respectively. The troponin T level had started to stabilize and the follow-up PET-CT (D325) indicated that activity had decreased (SUVmax 3.4) (). The patient is still receiving a prednisolone dose of 5 mg.
Figure 1. The schematic presentation illustrates the temporal behavior of the troponin T concentration throughout the follow-up period. D refers to days when the four treatment cycles (C1-4) were administered; the red vertical line indicates the reference value 15 ng/L for troponin T.
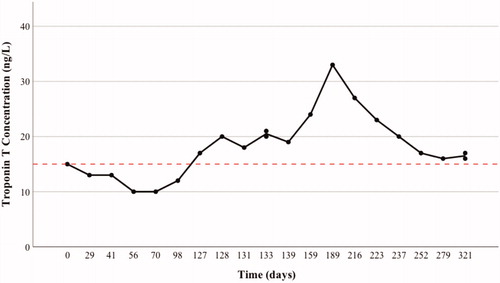
Figure 2. Initial FDG-PET-CT showed a focal uptake of radiotracer-marked glucose at the baseline (A) and at the follow-up (B). No findings were detected on cardiac magnetic resonance imaging (axial view image, (C). The SUVmax values were 5.4 and 3.4 at the baseline and the follow-up imaging, respectively (relative uptake color coding, (D)).
Discussion
Although several symptoms have been associated with ICI-induced myocarditis [Citation2], their absence may not be sufficient to rule it out. Mahmood et al. reported that levels of troponin and NT-pro-brain natriuretic peptide were elevated in 94% and 64% of patients with ICI-associated myocarditis, respectively [Citation4]; however, the specificity of cardiac biomarker elevation for the detection of ICI-associated myocarditis is unknown. In a recent review, Hu et al. proposed that cMRI would be superior to echocardiography in the detection of ICI-associated myocarditis [Citation2]. They postulated that cardiac FDG-PET-CT would be an alternative imaging modality to cMRI when MRI is contraindicated or not available. In accordance with the report of Mahmood et al. [Citation4], our case report illustrates that some cases of myocarditis may go undetected with cMRI. Therefore, if there remains a suspicion of ICI-associated myocarditis after a negative cMRI, FDG-PET-CT imaging may be beneficial in confirming the diagnosis.
Disclosure statement
Otso Arponen certifies that he has no conflicts of interest. Tanja Skyttä has received consultation fees and participated in advisory boards of Astra Zeneca, BMS, Boehringer Ingelheim, Incyte, Merck, MSD, Novartis, Pierre Fabre and Roche.
References
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ. 2018;360:k793.
- Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–868.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755.
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764.
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589.
