Abstract
Background: Pembrolizumab (P) and nivolumab (N) are commonly used therapies for advanced melanoma. However, their effectiveness has never been directly compared, leaving little guidance for clinicians to select the best therapy. Therefore, we sought to retrospectively compare the overall survival of patients with metastatic melanoma treated with front line P or N in the real-world setting.
Material and methods: This study included patients with advanced melanoma, diagnosed between 1 January 2011 and 31 July 2018, treated with frontline P or N who were included in a nationwide, longitudinal de-identified electronic health record (EHR)-derived database. Overall survival (OS) was estimated for each treatment group using Kaplan–Meier curves with a log-rank test. Comparison of OS was estimated using an inverse probability weighting model to reduce bias between the groups. The model was adjusted using age, sex, ECOG, LDH (elevated or not), BRAF (mutated or not), Kit (mutated or not), NRAS (mutated or not), PD-L1 expression (0% or greater), Body Mass Index, and primary site.
Results: 888 patients with advanced disease who received treatment with frontline P (n = 486) or N (n = 402) were identified. Median OS for all patients treated with P was 22.6 months (m) and was 23.9 m for those treated with N (p = 0.91). In the inverse probability weight analysis there was no difference in survival between patients treated with P or N 1.06 (95% CI 0.84–1.33).
Concluding Statement: In our retrospective, real-world analysis of patients with advanced melanoma, no statistical difference in OS was noted between patients treated with frontline P compared to N. This supports the current practice of choosing either P or N based on patient and provider preference.
Introduction
The treatment and prognosis of patients with high risk and advanced melanomas has changed dramatically over the past five years, largely due to the development of immune checkpoint inhibitors and antibodies to the programed cell death 1 receptor (anti-PD-1). The development of these drugs have improved the median survival of patients with metastatic/unresectable melanoma from 9 months [Citation1] (m) to over 36 m [Citation2]. Currently, both pembrolizumab and nivolumab are approved for metastatic/unresectable melanoma. Although these antibodies are both IgG4 subtype antibodies that target the PD-1 receptor, they bind at different epitopes on the receptor and with different affinities [Citation3] It is unknown if the differences in pharmacokinetics and dosing strategies between these two drugs affect the clinical outcome of patients.
Cross trial comparisons in melanoma demonstrate similar efficacy between pembrolizumab and nivolumab [Citation4,Citation5]. However, in metastatic non-small cell lung cancer (NSCLC), cross trial comparisons suggest different efficacies of these two drugs. Pembrolizumab has been shown to increase overall survival in patients with metastatic NSCLC with a PD-L1 expression of 1% or greater [Citation6]. However, nivolumab failed to show improvement in OS in patients with 5% or greater of PD-L1 expression in patients with metastatic NSCLC [Citation7]. Although the different outcomes in these two trials could be due to differences in the second line therapy that patients received in the chemotherapy arms, it does suggest a possible difference in efficacy between these two drugs.
There is currently no prospective data that has compared the effectiveness between these two medications, therefore physician and patient preference is typically used to choose the appropriate anti-PD-1 therapy. We sought to retrospectively compare the effectiveness of pembrolizumab and nivolumab in patients with advanced melanoma in a real-world patient population.
Material and methods
We conducted a real-world, retrospective study to compare the effectiveness of pembrolizumab and nivolumab for patients with advanced melanoma. We used the Flatiron Health database, a longitudinal, demographically and geographically diverse deidentified database derived from electronic health record (EHR) data. The database includes de-identified data from over 280 cancer clinics (∼800 sites of care), representing more than 2.1 million US cancer patients available for analysis. The patient-level data in the EHR include structured and unstructured data curated via technology-enabled abstraction. The Copernicus Institutional Review Board (IRB) provided approval, with waiver of informed consent, of the research dataset prior to study conduct. The University of Utah IRB reviewed the study protocol and granted an exemption.
The study included patients diagnosed with advanced melanoma between 1 January 2011 and 31 July 2018, who received frontline therapy with either pembrolizumab or nivolumab. Patients with incomplete clinic records or insufficient follow up, fewer than 30 days, from initiation of frontline therapy were excluded. Patients treated in the frontline setting with multi-agent combination therapies that included pembrolizumab or nivolumab were excluded.
Overall survival (OS), based on EHR documentation plus linkage to external data sources [Citation8], from the initiation of frontline therapy was compared between those treated with frontline pembrolizumab and nivolumab. As progression data was not readily accessible for all patients in the dataset, a potential surrogate for progression free survival, time to next line therapy or death (TTNTD), was also compared between those treated with pembrolizumab versus nivolumab. TTNTD was calculated in a hierarchical manner, where for patients who received second line therapy, TTNTD was calculated from the initiation of first line therapy to the initiation date of second line therapy. For patients who died without receiving second line therapy, TTNTD was calculated from the initiation of first line therapy to the date of death. Patients who were alive without receiving second line therapy at the time of analysis were censored at last known follow up.
The OS and TTNTD for patients treated with pembrolizumab and nivolumab were compared using Kaplan Meier curves and log-rank analysis. Cox regression analysis was also used to compare the OS and TTNTD for these populations. 3 separate Cox regression models were performed: an unadjusted model, an adjusted model, and an inverse probability weighting model. The adjusted model was adjusted for age at initiation of treatment, sex, pretreatment ECOG performance status, pretreatment LDH (value greater than upper limit of normal or not), BRAF mutational status (identification of a targetable mutation or not), KIT mutations status, NRAF Mutations status, PD-L1 positivity (0% or greater), pretreatment Body Mass Index (BMI) and primary site of disease. Pretreatment was defined as being documented within 30 days of starting frontline therapy. For the inverse probability weighting model, we fitted a logistic regression model to estimate the probability of the treatment for each patient, and used the inverse predicted probability as a weight in the Cox regression model. The logistic regression model for the treatment was adjusted for the same covariates above.
Results
7,650 patients with melanoma were identified within the Flatiron Health database. 3,743 patients received systemic therapy for advanced disease, of whom 1,090 received treatment with either frontline pembrolizumab or nivolumab. 202 patients were excluded due to insufficient data or follow up. The final dataset included 486 patients who received frontline pembrolizumab and 402 patients treated with frontline nivolumab.
The median age for patients included in our study was 74. Median ECOG performance status and pretreatment LDH was 1 and 236 (units/liter), respectively. Patients treated with frontline pembrolizumab were older and less likely to receive second line therapy as compared to those treated with nivolumab. There was no difference between those treated with pembrolizumab and nivolumab in regard to sex, performance status, pretreatment LDH, BMI, mutational status for BRAF, NRAS, or KIT, PD-L1 positivity, primary site of disease, or vital status at last follow up ().
Table 1. Patient demographics for all patients.
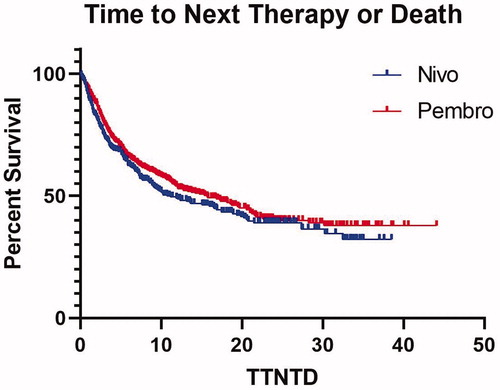
With a median follow up of 17.3 months, median OS and TTNTD for all treated patients were 22.6 m [interquartile range (IQR) 6.8-not reached (NR)] and 13.6 m (IQR 3.4-NR), respectively. The median OS for patients treated with frontline pembrolizumab was 22.6 m (IQR 6.8-NR), while median OS for those treated with nivolumab was 23.9 m (IQR 6.8–39.5), p = 0.91 (). Median TTNTD for patients treated with frontline pembrolizumab or nivolumab was 15.7 m (IQR 3.4–NR) and 10.8 m (IQR 3-NR), p = 0.16 ().
Figure 1. Overall survival of patients with advanced melanoma treated with frontline pembrolizumab or nivolumab.
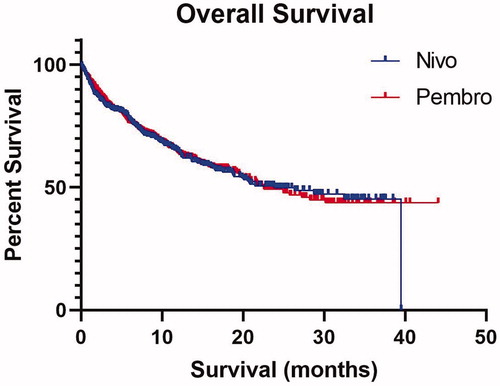
Figure 2. Time to Next Line of Therapy or Death (TTNTD) for patients with advanced melanoma treated with frontline pembrolizumab or nivolumab.
In the inverse probability weighting model, the hazard ratios (HR) for OS and TTNTD for receiving pembrolizumab, as compared to nivolumab, were 1.06 (95% CI 0.84–1.33) and 0.92 (95% CI 0.76–1.12), . The HRs did not vary between the different analyses and all included one within the 95% CI.
Table 2. Hazard Ratio for Overall Survival (OS) and Time to Next Therapy or Death (TTNTD) for receiving Pembrolizumab versus Nivolumab.
Discussion
In this retrospective, real-world analysis of patients with advanced melanoma treated with frontline anti-PD-1 therapy, there was no meaningful difference between TTNTD or OS for patients treated with frontline pembrolizumab as compared to nivolumab, before or after inverse probability weighting.
Median OS for all patients included in our analysis was 22.6 months. This is lower than reported median OS from clinical trials for frontline therapy for nivolumab and pembrolizumab, roughly 36.9 and 38.7 m [Citation2,Citation4]. One reason for this difference could be that there is a shorter median duration of follow up in our study, as compared to the clinical trials. Another reason could be the difference in the patient populations between prospective clinical trials and our real world dataset. For example, both clinical trials using pembrolizumab and nivolumab excluded patients with active CNS disease and performance status of 2 or greater. However, 19 percent of patients, for whom ECOG data was available, in our analysis had a poor performance status. Additionally, site of metastatic disease is not recorded in the Flatiron Health database. Therefore, we do not know what percentage of patients had active CNS disease, however there is presumably a proportion of patients in the database that do. Interestingly, despite inferior overall survival noted in our dataset, as compared to large clinical trials, little difference was noted between the TTNTD in this study and PFS in the clinical trials. Median TTNTD for patients included in this analysis was 13.6 m, while reported median PFS for nivolumab and pembrolizumab was 6.9 [Citation4] and 11.2 m [Citation2], respectively. One reason that might explain the similarity of TTNTD in this study with PFS in the clinical trials, but inferior OS for patients in our study is an increased proportion of BRAF mutations for patients included in the clinical trials. In our study sample, there was roughly a 10% lower presence of actionable BRAF V600 mutations. Therefore, a higher percentage of patients in our study had fewer effective treatment options available for their advanced melanoma after progression as compared to those included in prospective clinical trials.
Limitations of this study include the potential imperfection of TTNTD as a surrogate for PFS, and the imperfection of inverse probability weighting as a method of retrospectively balancing treatment arms. Another limitation of this study is the lack of information in the database in regards to local therapies, such as metastectomies and radiation therapy, both of which could impact TTNTD and OS.
Conclusion
In the multiple different analyses performed in this study, we found no difference between the effectiveness of pembrolizumab and nivolumab. Therefore, our study supports the current clinical practice of choosing either drug based on patient and clinician preference.
Disclosure statement
KFG has advised for BMS (within last year), Novartis (2 years ago), Array (within last year), Castle Biosciences (>3 years ago) – all less than 5k annually. SP received institutional research funding from Merck and Takeda. JRH is contracted with Ebix publishers to perform audio reviews of general surgery published articles. This has no relationship to melanoma research. JCM has advised for Caris Life Sciences. GW and SVC have no disclosures.
References
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526.
- Long GV, Schachte J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (IPI)-naive advanced melanoma in KEYNOTE-006. ASCO Annual Meeting. JCO. 2018;36(15_suppl):9503–9503.
- Fessas P, Lee H, Ikemizu S, et al. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44(2):136–140.
- Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492.
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–1862.
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415–2426.
- Curtis MD, Griffith SD, Tucker M, et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. 2018;53(6):4460–4476.
