Abstract
Purpose: The primary aim of the study was to assess the association between having a radiotherapy (RT) department on-site at the surgical centre and the performed postoperative treatment strategy for prostate cancer (PCa) patients. According to the current international guidelines, adjuvant radiotherapy (ART) or a regular prostate-specific antigen (PSA)-based follow-up with (early) salvage radiotherapy ((e)SRT) if needed is recommended in case of adverse pathological characteristics.
Material and methods: Prospective data on consecutive robot-assisted radical prostatectomy (RARP) patients in Belgium from 2009 to 2016 were identified in the Belgian Robotic-Assisted-Laparoscopic-Prostatectomy (Be-RALP) database. Multivariable regression was used to evaluate patient- and facility-related factors associated with postoperative radiation treatment.
Results: 2072 patients undergoing a RARP, suffering at least one of the following adverse pathological features, i.e., extracapsular extension (ECE), seminal vesicle invasion (SVI) or positive section margins (PSM), and with registered follow-up until 24 months were enrolled. After RARP, ART was applied to 9.1% and (e)SRT to 12.6% of the patients. Multivariable analysis demonstrated that patients were more likely to receive ART or (e)SRT if they were operated in a hospital with a RT department on-site (odds ratio, ART: 1.49 [1.07-2.07]; (e)SRT: 1.55 [1.16-2.06]). Furthermore, the presence of higher tumour category (T-category) and/or PSM on final pathology was associated with a higher chance of getting ART and (e)SRT (p < .01).
Conclusion: Variations in ART and (e)SRT are not only driven by patient-related characteristics. In our nationwide cohort, the availability of a RT department on-site at the surgical centre was found to be an independent predictor for ART and (e)SRT, with a 1.5 times higher odds of receiving postoperative RT during the first 24 months after surgery.
Introduction
Prostate cancer (PCa) is the most common non-skin malignancy and an important cause of cancer-related mortality in men in Europe [Citation1]. Over 9000 new patients are diagnosed with PCa in Belgium each year [Citation2]. During the past decades, a significant increase in the use of radical prostatectomy (RP) is noticed for men diagnosed with intermediate and high risk PCa [Citation3,Citation4]. In case of presence of adverse pathological characteristics at the RP specimen, the risk of biochemical recurrence mounts to 60%. These adverse pathological characteristics include extracapsular extension (ECE; pT3a), seminal vesicle invasion (SVI; pT3b), presence of positive section margins (PSM) or a combination of these characteristics [Citation5,Citation6].
The current role of post-RP radiotherapy (RT) in these patients remains a matter of debate. Three randomised controlled trials investigated the added value of adjuvant radiotherapy (ART) vs observation in patients with high risk pathological features [Citation7–9]. Both the European Organisation for Research and Treatment of Cancer (EORTC) 22911 trial and the Arbeitsgemeinschaft Radiologische Onkologie (ARO) 96-02 demonstrated a benefit in progression-free survival (PFS) after 10-year follow-up, but neither could show a significant difference in metastasis-free survival (MFS) nor overall survival (OS) [Citation7,Citation8]. Only the Southwest Oncology Group (SWOG) 8794 trial showed a benefit for MFS and OS after 10-year follow-up for the group treated with ART [Citation9]. Based on these three randomised trials, the consensus guidelines of the European Association of Urology (EAU) recommend ART in patients with ≥ pT3 PCa and/or PSM and undetectable prostate-specific antigen (PSA) following a RP [Citation10]. Furthermore, the American Urological Association & American Society for Radiation Oncology (AUA-ASTRO) guidelines advise to offer ART after discussion with the patient in this particular patient group (grade A recommendation) [Citation11].
Both the concern of the impact on urinary continence and the risk of overtreatment resulted in an ambivalent attitude of urologists and patients towards the use of ART. Therefore, another approach to treat high risk PCa patients after RP is (early) salvage radiotherapy ((e)SRT). This strategy is based on monitoring the PSA level and once it becomes detectable, offering (e)SRT. The currently available evidence supporting (e)SRT is based on retrospective trials only [Citation12–14]. Nevertheless, this alternative treatment strategy was already implemented into the 2010 version of the EAU guidelines. Currently, primary endpoint results of three prospective randomised trials comparing ART with (e)SRT are awaited [Citation15–17]. Preliminary results from a first, predefined, meta-analysis (ARTISTIC collaboration), including these three prospective randomised trials, suggest that (e)SRT and ART offer similar outcomes regarding 5-year event-free survival [Citation18].
Despite the general recommendation for ART discussion by international consensus guidelines, ART rates remain low. Previous reports demonstrated that the use of ART did not increase after publication of the results of these randomised trials [Citation19,Citation20].
The purpose of the present study was to analyse the patterns of postoperative RT after robot-assisted radical prostatectomy (RARP) for PCa in Belgium. In addition to the investigation of potential influences due to the availability of a RT department on-site on the administration of either ART or (e)SRT, further insights were sought into medical factors associated with practice patterns. Moreover, the influence of guideline changes over time concerning (e)SRT on these treatment patterns was evaluated.
Material and methods
Study design and data source
From October 2009, hospitals and urologists performing RARP in Belgium were reimbursed for this specific technique by the National Institute for Sickness and Invalidity Insurance (Rijksinstituut voor Ziekte- en Invaliditeitsverzekering/Institut National d’Assurance Maladie Invalidité (RIZIV/INAMI)), but with an obligatory ‘clinically oriented’-registration at the Belgian Cancer registry (BCR). In collaboration with the Belgian Association of Urology (BAU), the Belgian Robotic-Assisted-Laparoscopic-Prostatectomy (Be-RALP) Registry project was started. Centres wishing to participate in this project had to sign an agreement with the RIZIV/INAMI and register new diagnosis data and follow-up data for patients who were treated with RARP in their institution. A 1/3/12/24-month follow-up was requested. The primary goal of the Be-RALP project was to assess the outcome and quality of care in patients treated by a RARP. The data were electronically registered in a prospective manner, transmitted to the Belgian Cancer Registry and handled in accordance with the Belgian Privacy Law (Law of 8 December 1992). Patient registration for index surgery was closed on February 29, 2016. Twenty-five academic and non-academic centres performed RARP procedures in Belgium and participated in patient data gathering. Follow-up records were completed up to August 31, 2018. For the present study, we collected the following data from the Be-RALP database for each case: age, surgery date, tumour, node, metastasis (TNM)-category, International Society of Urological Pathology (ISUP) group, section margin status and postoperative, 12-month and 24-month follow-up data. These follow-up data include a PSA measurement and information concerning any postoperative therapy initiated. The Be-RALP database also specified the radiation dose and the start date of any postoperative treatment.
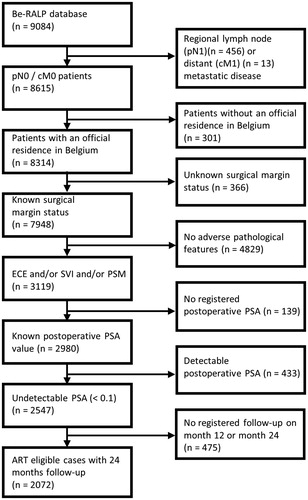
In this retrospective cohort study, we included patients with at least one of the three previously described adverse features (ECE, SVI and PSM) and an undetectable postoperative PSA value from the Be-RALP database. Exclusion criteria were missing key data (e.g., missing section margin status, missing postoperative PSA level and missing follow-up registrations), diagnosis of regional lymph node or distant metastasis at staging and not living in Belgium because of a potential deviant external follow-up. A cohort of 2072 patients was retained to evaluate the postoperative practice patterns after RARP ().
Definitions
Pathological evaluation was performed according to the ISUP grade grouping system [Citation21,Citation22] and staging was described with the pathological TN (pTN) classification of the Union for International Cancer Control (UICC) 7th edition. PSMs were defined as tumour tissue, at least 1 malignant gland, identified on the inked surface of the specimen. The correctness of data entry for pathological variables by the participating centres was cross-checked with individual patient data by the Belgian Cancer Registry. These individual patient data are collected directly and independently from the pathologists of each hospital in Belgium.
The PSA level detection limits varied among centres and over time. We considered PSA level as undetectable if it was less than 0.1 ng/mL. The postoperative PSA level was defined as the PSA registered at the first follow-up moment, situated 1 to 3 months after RARP. If a further PSA decrease was noticed during this 3-month period without treatment intervention, the lowest PSA level was considered as postoperative PSA.
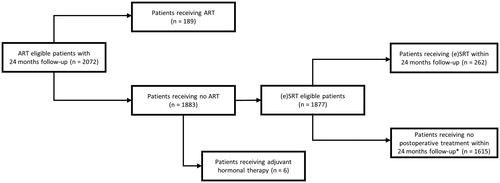
ART was defined as external beam RT delivered within 6 months after surgery in patients with an undetectable postoperative PSA. Furthermore, (e)SRT was defined as RT delivered more than 6 months after RARP, or RT delivered within 6 months after RARP with a detectable PSA value (≥ 0.1 ng/mL) when RT was administered, following an earlier undetectable PSA value. Both ART and (e)SRT were retained for analysis if a relevant curative radiation dose (60–80 Gy) was delivered on the prostate bed. Patients who received ART or adjuvant hormonal therapy after surgery were excluded for the (e)SRT analysis ().
Figure 2. Patient flow diagram of the study. *A 24-month follow-up was requested. ART: adjuvant radiation therapy; (e)SRT: (early) salvage radiation therapy.
The 25 participating hospital centres were grouped based on their volume. Low volume centres (n = 13) were defined as performing a mean of less than 50 RARPs each year, whereas centres which performed a mean of more than 100 RARPs each year were defined as high volume centres (n = 3). Hospital centres with 50–100 RARPs each year were categorised as medium volume centres (n = 9). Furthermore, for each of the 25 participating RARP centres, we identified the availability of a RT department on-site (12/25). For 2 centres, a RT department was opened during the registration period (Oct 2009–Feb 2016). These centres were classified as ‘radiation department on-site’ or ‘no radiation department on-site’ based on the number of patients that was registered for the Be-RALP database from the moment the RT department was opened till the end of the registration period: if the number of registered patients during that period was higher than the number of registered patients before the opening of the RT department, the centre was considered as ‘radiation department on-site’.
Statistical analysis
Descriptive statistics of categorical variables focus on frequencies and proportions. Univariable and multivariable logistic regression analysis for patient-related and facility-related parameters to evaluate the rate of ART and (e)SRT use were performed. Patient-related variables for multivariable analysis were selected after significance in univariable analysis. Associations were considered statistically significant if p < .05. A Wilcoxon rank sum test was employed to compare the PSA level distribution between the ‘radiation department on-site group’ and the ‘no RT department on-site group’. Data were analysed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC, USA).
Results
Patients
Twenty-five hospitals across Belgium participated in patient data gathering from October 2009 to February 2016. During this period, 9084 unique patients were registered in the Be-RALP database. Among these, a subgroup of 2072 patients with at least one risk factor, i.e., ECE, SVI or PSM, an undetectable postoperative PSA value and registered follow-up visits 12 and 24 months after RARP was selected for further analysis ().
shows the characteristics of the selected patient cohort (n = 2072). The mean age at surgery was 65 year [IQR, 60–69 year]. Among the included patients, 36.7% underwent a RARP in a hospital without RT facility. During the median 2-year follow-up after RARP, 21.8% of the patients with at least 1 risk factor underwent postoperative RT delivered in either an adjuvant (9.1%) or (early) salvage (12.6%) setting. The yearly evolution in administered postoperative RT strategy after RARPs performed from October 2009 to February 2016 is shown in .
Figure 3. Temporal Trends in Post Robot-Assisted Radical Prostatectomy (RARP) Radiation Therapy in Belgium. *Either ART treated patients (n = 189), or adjuvant hormonal therapy treated patients (n = 6) are not eligible for (e)SRT according to the definition of (e)SRT. ART: adjuvant radiation therapy; (e)SRT: (early) salvage radiation therapy; RARP: robot-assisted radical prostatectomy; RT: radiation therapy
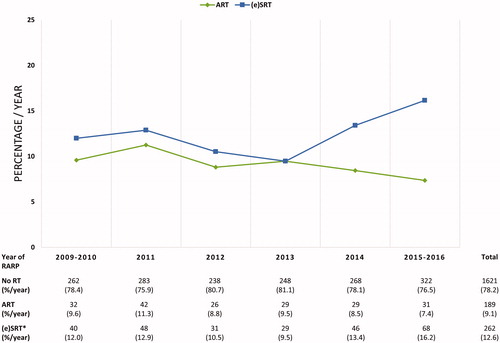
Table 1. Characteristics of 2072 patients registered in the Be-RALP database with at least one of the following adverse pathological features (extracapsular extension (ECE), seminal vesicle invasion (SVI) or positive section margins (PSM)) and 24 months follow-up.
Adjuvant radiotherapy
The univariable and multivariable logistic regression findings concerning the use of ART are summarised in . Univariable logistic regression results show higher ART rates for patients with adverse pathological characteristics, including a higher pathological T-category (pT3a vs pT2 and pT3b-4 vs pT2) (odds ratio, 1.49 [95% CI, 1.02–2.18]; 3.31 [95% CI, 2.16–5.08]) and the presence of PSMs (odds ratio, 2.13 [95% CI, 1.51–3.01]). An additional effect is seen if a higher pT-category coincides with PSMs. The apparent association between a higher ISUP grading on the RARP specimen (ISUP grade ≤ 3 vs ISUP grade ≥ 4) and ART noted in univariable analysis (odds ratio, 1.56 [95% CI, 1.09–2.22]), became non-significant in the multivariable model (odds ratio, 1.21 [95% CI, 0.83–1.77]). Regarding the facility-related parameters as a predictor of receiving ART, the availability of a RT centre on-site at the surgical centre became a significant predictor (odds ratio, 1.49 [95% CI, 1.07-2.07]) in the multivariable analysis. shows the absolute and percentage distribution of ART after RARP according to the availability of a RT department on-site at the surgery centre. In our study, there was no association found for age (<70 vs ≥70 year) and hospital RARP volume with the ART rate.
Table 2. Factors associated with post RARP radiation therapy regarding ART (n = 2072) and (e)SRT (n = 1877; not including either ART treated patients (n = 189), or adjuvant hormonal therapy treated patients (n = 6)).
(Early) salvage radiotherapy
The subgroup of non-adjuvant treated patients (n = 1877) was retained to analyse the use of (e)SRT in our cohort. Consistent with the proven predictors for ART, a higher pathological T-category (pT3b-4 vs pT2) (odds ratio, 1.58 [95% CI, 1.08–2.32]) and the presence of PSMs (odds ratio, 2.17 [95% CI, 1.62–2.91]) were also retained as predictors of the need for (e)SRT. Similarly, a higher ISUP grading on the RARP specimen (ISUP grade ≤ 3 vs ISUP grade ≥ 4) is not retained as a predictor for the need of (e)SRT during the first 24 months after RARP by the multivariable model (odds ratio, 1.36 [95% CI, 0.96-1.92]). The availability of a RT facility located in the hospital where surgery was performed is also in the salvage setting associated with increased (e)SRT administration by multivariable analysis (odds ratio, 1.55 [95% CI, 1.16–2.06]). There was no significant difference in follow-up PSA value distribution between the ‘radiation therapy on-site group’ and the ‘no RT department on-site group’ at 12 months (p = 0.46) and 24 months (p = 0.12) follow-up. For (e)SRT, an association was found with the hospital RARP volume, with lower (e)SRT rate in medium (odds ratio, 0.63 [95% CI, 0.47–0.84]) and low volume centres (odds ratio, 0.64 [95% CI, 0.45–0.91]) compared to high volume centres. An association between the age at surgery (<70 vs ≥70 year) and (e)SRT administration was not found. Details about the univariable and multivariable analyses are depicted in . The absolute and percentage distribution of (e)SRT after RARP according to the availability of a RT department on-site at the surgery centre is depicted in .
Discussion
Although the Belgium healthcare system is noted for being one of the most accessible in Europe with low travel distances to well-equipped RT facilities [Citation23,Citation24], our findings demonstrate a statistically significant difference in the administration of postoperative RT after RARP depending on the availability of a RT department on-site at the RARP centre. Our analysis shows that the odds of receiving either ART or (e)SRT is 1.5 times higher when a prior RARP was performed in a hospital centre with a RT department on-site. These results were adjusted for the number of adverse pathological features, but not for age as there was no significant association in the univariable analysis and no interactions are expected between age and the adverse pathological features. Many factors could explain this difference in RT delivery. Fowler et al. have previously demonstrated the existence of a bias in treatment strategy favouring known clinical pathways by urologists and radiation oncologists in the treatment of PCa [Citation25]. Concordant with these findings, it was previously demonstrated that actual RT delivery is lower than the evidence-based optimum [Citation26]. The inclusion period of the Be-RALP database spans from October 2009 to February 2016. It should be noted that during this period, close cooperation between several hospitals in so-called hospital networks was still in its infancy in Belgium. In the meantime, a national agreement was reached and the organisation of hospital networks in Belgium progressed well. The implementation of such hospital networks including at least one RT department will lead to harmonisation of the postoperative RT strategy after RARP.
In addition to the influence of the availability of a RT facility on-site at the RARP centre on the performed postoperative treatment strategy, we investigated the influence of guideline changes over time on it. Based on multiple international guidelines, immediate postoperative RT after RARP should be discussed in patients with unfavourable pathological outcomes, defined as ECE, SVI and PSMs. However, according to the EAU guidelines, (e)SRT in case of rising PSA is considered as an acceptable alternative treatment strategy for these ART-eligible patients and was mentioned in the EAU guidelines since 2010. Although not significant yet, a gradual shift from ART towards (e)SRT seemed apparent within the postoperative management of RARP patients with adverse pathological characteristics.
Notably, higher ART and (e)SRT rates were associated with higher pT-category and the presence of PSMs. These rates were even higher in case of multiple unfavourable pathological features. These findings are consistent with earlier reports [Citation27,Citation28]. The disappearance of a significant association between higher ISUP grades and ART or (e)SRT in the multivariate model may be due to covariation between high pT-categories and high ISUP grades. Furthermore, although the EORTC 22911 trial reported a detrimental outcome for clinical progression-free survival after immediate irradiation in 70 years or older patients, ART rates were similar in the patient cohort until 70 years compared to the over 70 s group [Citation7].
Our study has several limitations. First, while patients were followed for 24 months after RARP, (e)SRT rates may still increase over time. Furthermore, the interpretation of our results is limited by the information available in the Be-RALP database. We were unable to adjust our results for certain factors such as patient preferences, the extent of PSM and ECE and the recovery of genitourinary toxicities after surgery. Follow-up PSA measurements at month 12 and 24 were used as a surrogate for the PSA trend over the 2-year period. Due to the set-up of the Be-RALP database, our study cohort includes only RARP patients. However, we do not expect that the postoperative approach is dependent on the type of surgical technique used. Finally, despite the fact that this is a comprehensive, prospective, and mandatory registration, only pathological data but not follow-up data were cross-checked with individual patient data by the Belgian Cancer registry. However, financial consequences for the registering hospitals were linked to underreporting of follow-up data.
Despite these limitations, results of our study provide valuable insight into the determinants of applying radiation therapy as adjuvant or (early) salvage treatment for PCa patients. Additional research is needed to investigate whether more intensive multidisciplinary collaboration between various hospitals, in which a radiation-oncologist is closely involved in the therapeutic decision-making process, may lead to a more homogenous oncologic treatment policy within one country. Efforts should be made to reduce inter-centre practice variation. Furthermore, the choice between ART and (e)SRT is currently the subject of three prospective multicentre trials (RADICALS, RAVES, and GETUG-17) randomising patients with adverse pathologic characteristics to ART versus observation ± (e)SRT [Citation15–17]. The primary endpoint results of these trials are eagerly awaited for. However, results from a first, predefined, meta-analysis (ARTISTIC collaboration) suggest that (e)SRT and ART offer similar outcomes regarding 5-year event-free survival [Citation18]. As all patients with adverse pathological factors are eligible for ART, but only the subgroup with biochemical recurrence receives (e)SRT in the ARTISTIC collaboration, a majority of patients are saved from RT-induced side-effects. Considering our results and the ARTISTIC meta-analysis results, the application and timing of (e)SRT can be considered as a quality indicator for PCa care.
To conclude, variations in ART and (e)SRT are not only driven by patient-related characteristics. In our nationwide cohort, the availability of a RT department on-site at the surgical centre was found to be an independent predictor for ART and (e)SRT, with a 1.5 times higher odds of receiving postoperative RT during the first 24 months after surgery.
Acknowledgements
We thank the Belgian Robotic‐Assisted Laparoscopic Prostatectomy Registry (Be-RALP) Steering Committee, consisting of Dr. Filip Ameye, Prof. Dr. Steven Joniau and Prof. Dr. Thierry Roumeguère (Belgian Association of Urology (BAU) Oncology Working Group); Dr. Peter Dekuyper, Dr. Thierry Quackels and Dr. Ben Van Cleynenbreugel (Belgian Laparoscopic Urology Group (BLUG)); and the Belgian Cancer Registry.
We thank all the members of the Belgian Robotic‐Assisted Laparoscopic Prostatectomy Registry (Be-RALP) and all surgeons participating in the study: M. Claessens and K. Hente (AZ Klina, Brasschaat, Belgium); T. Adams, G. Boeckx, H. Vandeursen, Dr. Eric Vergauwe, and Dr. Gustaaf Witters (Campus Sint‐Augustinus, GZA Ziekenhuizen, Antwerp, Belgium); C. Assenmacher and J. Benijts (Cliniques de l’Europe, Brussels, Belgium); A. Corbusier and C. Chatzopoulos (CHIREC, Brussels, Belgium), R. van Velthoven and A. Peltier (Institut Jules Bordet, Brussels, Belgium); K. Entezari and C. Entezari (CHU Saint Pierre, Brussels, Belgium); G. Martens (AZ Jan Portaels, Vilvoorde, Belgium); J. Ampe, C. Ghysel, W. Van Haute, P. Van Oyen, and B. Verlinde (AZ Sint‐Jan, Brugge‐Oostende, Belgium); L. Vandenbussche (AZ Sint Lucas, Brugge, Belgium); I. Billiet, K. Lesage, and P. Werbrouck (AZ Groeninge, Kortrijk, Belgium); W. Marchand, H. Van Der Eecken, and J. Vanhoucke (AZ Delta, Heilig Hart Ziekenhuis Roeselare, Menen, Belgium); J. Darras (AZ Damiaan, Oostende, Belgium); K. Decaestecker, B. De Troyer, N. Lumen, F. Poelaert and C. Van Praet (Universitair Ziekenhuis, Ghent, Belgium); L. Merckx (AZ Sint Lucas, Ghent, Belgium); F. Ameye, P. Dekuyper and A. Van Baelen (AZ Maria Middelares, Ghent, Belgium); G. Van Holderbeke, T. Van Erps and C. Verbaeys (AZ Jan Palfijn, Ghent, Belgium); C. Peeters, P. Schoonooghe, and M. Stragier (AZ Sint Elisabeth, Zottegem, Belgium); D. Ost, K. Maes and K. Vander Eeckt (AZ Sint Blasius, Dendermonde, Belgium); F. Peeren and B. Rappe (Algemeen Stedelijk Ziekenhuis, Aalst, Belgium); A. Mottrie and G. de Naeyer (Onze‐Lieve‐Vrouwziekenhuis, Aalst‐Asse‐Ninove, Belgium); M. Naudin (CHU Ambroise Paré, Mons, Belgium); O. Lavergne and H. Nicolas (CHR de la Citadelle, Liège, Belgium); B. Van Cleynebreugel (UZ Leuven, Leuven, Belgium); M. Vanden Bossche and T. Quackels (Cliniques Universitaires de Bruxelles - Hôpital Erasme, Brussels, Belgium) and F. Lorge (Cliniques Universitaires de Mont Godinne, Yvoir, Belgium).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Belgian Cancer Registry. Cancer fact sheet prostate cancer Belgium 2016. Available from: https://kankerregister.org/Cancer_Fact_Sheets
- Hager B, Kraywinkel K, Keck B, et al. Increasing use of radical prostatectomy for locally advanced prostate cancer in the USA and Germany: a comparative population-based study. Prostate Cancer Prostatic Dis. 2017;20(1):61–66.
- Albisinni S, Joniau S, Quackels T, et al. Current trends in patient enrollment for robotic-assisted laparoscopic prostatectomy in Belgium. Cancer. 2017;123(21):4139–4146.
- Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28(3):555–565.
- Spahn M, Weiss C, Bader P, et al. Long-term outcome of patients with high-risk prostate cancer following radical prostatectomy and stage-dependent adjuvant androgen deprivation. Urol Int. 2010;84(2):164–173.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018–2027.
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95. Trial Eur Urol. 2014;66(2):243–250.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629.
- Pisansky TM, Thompson IM, Valicenti RK, et al. Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J Urol. 2019;202(3):533–538.
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. JCO. 2007;25(15):2035–2041.
- Wiegel T, Lohm G, Bottke D, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome—results of a retrospective study. Int J Radiat Oncol. 2009;73(4):1009–1016.
- Ost P, De Troyer B, Fonteyne V, et al. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol. 2011;80(5):1316–1322.
- Parker C, Clarke N, Logue J, et al. RADICALS (radiotherapy and androgen deprivation in combination after local surgery). Clin Oncol. 2007;19(3):167–171.
- Richaud P, Sargos P, Henriques de Figueiredo B, et al. GETUG AFU 17. Radiothérapie postopératoire des cancers de la prostate. Cancer/Radiothérapie. 2010;14(6–7):500–503.
- Pearse M, Fraser-Browne C, Davis ID, et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the radiotherapy – adjuvant versus early salvage (RAVES) trial. BJU Int. 2014;113(Suppl 2):7–12.
- Vale CL, Brihoum M, Chabaud S, et al. Adjuvant or salvage radiotherapy for the treatment of localised prostate cancer? A prospectively planned aggregate data meta-analysis. Ann Oncol. 2019;30:883.
- Sheets NC, Hendrix LH, Allen IM, et al. Trends in the use of postprostatectomy therapies for patients with prostate cancer: a surveillance, epidemiology, and end results medicare analysis. Cancer. 2013;119(18):3295–3301.
- Sineshaw HM, Gray PJ, Efstathiou JA, et al. Declining use of radiotherapy for adverse features after radical prostatectomy: results from the national cancer database. Eur Urol. 2015;68(5):768–774.
- Epstein JI, Allsbrook WC, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29(9):1228–1242.
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2015;40(2):1–52.
- Björnberg A, Phang AY. Euro Health Consumer Index Report 2018. Avaiable from: https://healthpowerhouse.com/publications/
- Grau C, Defourny N, Malicki J, et al. Radiotherapy equipment and departments in the European countries: final results from the ESTRO-HERO survey. Radiother Oncol. 2014;112(2):155–164.
- Fowler FJ, Jr, Collins MM, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222.
- Lievens Y, De Schutter H, Stellamans K, et al. Radiotherapy access in Belgium: how far are we from evidence-based utilisation.? Eur J Cancer. 2017;84:102–113.
- Jin CJ, Hanna TP, Cook EF, et al. Variation in radiotherapy referral and treatment for high-risk pathological features after radical prostatectomy: results from a population-based study. Clin Oncol. 2018;30(1):47–56.
- Hawken SR, Spratt DE, Qi J, et al. Utilization of salvage radiation therapy for biochemical recurrence after radical prostatectomy. Int J Radiat Oncol. 2019; 104(5):1030–1034.