Abstract
Background
Radiotherapy is an emerging treatment strategy for nodal oligorecurrent prostate cancer (PCa) patients. However, large heterogeneities exist in the RT regimens used, with series reporting the use of elective nodal radiotherapy (ENRT) strategies and others the delivery of focal treatments to the relapsing nodes with Stereotactic Body Radiotherapy (SBRT). In this systematic review of the literature we compared the oncological outcomes and toxicity of the different RT regimens for nodal oligorecurrent PCa patients, with the aim of defining the optimal RT target volume in this setting.
Methods
We performed a systemic search on the Pubmed database to identify articles reporting on the use of ENRT or SBRT for oligometastatic PCa with nodal recurrence.
Results
Twenty-two articles were analyzed, including four prospective phase II trials (3 with SBRT and 1 with ENRT). Focal SBRT, delivered with an involved node, involved site, and involved field modality, was the most commonly used strategy with 2-year progression-free survival (PFS) rates ranging from 16 to 58% and a very low toxicity profile. Improved PFS rates were observed with ENRT strategies (52–80% at 3 years) compared to focal SBRT, despite a slightly higher toxicity rate. One ongoing randomized phase II trial is comparing both modalities in patients with nodal oligorecurrent PCa.
Conclusions
With a large variability in patterns of practice, the optimal RT strategy remains to be determined in the setting of nodal oligorecurrent PCa. Ongoing randomized trials and advances in translational research will help to shed light on the best management for these patients.
.
Introduction
After curative treatment of localized prostate cancer (PCa), up to 50% of the patients with high-risk disease can experience a biochemical relapse [Citation1]. Restaging by modern metabolic imaging modalities allows visualization of clinical relapse at very low prostate specific antigen (PSA) levels, with the majority of the patients harboring an oligorecurrent disease, defined as up to 3 or 5 lesions, mainly located in the pelvis [Citation2–4].
Systemic therapy with androgen deprivation therapy (ADT) remains the cornerstone treatment of metastatic PCa patients relapsing after a definitive treatment of the primary [Citation5]. However, considering the better overall prognosis of patients with nodal oligorecurrence compared to those with bone and/or visceral metastases [Citation6,Citation7], radical salvage treatments including salvage lymph node dissection (sLND) or radiotherapy (RT) have been proposed and implemented to delay the use of palliative ADT or even cure these patients [Citation8–10]. Restaging imaging is therefore crucial to differentiate these patients in order to adapt the therapeutic strategy.
The optimal imaging modality for restaging is not well defined. The version 4.2019 of the National Comprehensive Cancer Network (NCCN) guidelines questions the utility of conventional imaging (bone scan or computed tomography) for men with early PSA recurrence after radical prostatectomy (RP) [Citation11]. Multiparametric magnetic resonance imaging (MRI), positron emission tomography/computed tomography (PET-CT) or PET-MRI with choline and/or fluciclovine tracers can be proposed as preferred imaging modalities in this setting, while use of 68Ga prostate specific membrane antigen (PSMA) is restricted to clinical trials or registries. On the contrary, in the 2019 update of the European Association of Urology (EAU) guidelines, PSMA PET-CT is recommended as the preferred restaging modality for PSA recurrences after RP > 0.2 ng/ml if the results will influence subsequent treatment decisions [Citation5]. However, despite the emerging role of PSMA PET-CT as standard restaging modality compared to other tracers [Citation12], with promising detection rates even for low PSA values [Citation13] and a potential treatment change in up to 60% of the cases [Citation14], its sensitivity in detecting the nodal disease in sLND series remains limited to a 60% rate [Citation15–17].
In analogy with the open issues existing on the best restaging imaging modality and the inherent limitations of each technique, the optimal RT regimen for nodal oligorecurrent PCa patients is also not yet defined, with large treatment variability between centers and no consensus on volumes, doses and techniques [Citation18,Citation19]. Salvage RT for nodal recurrences can be delivered either by covering prophylactically the bilateral pelvic drainage regions (elective nodal radiotherapy, ENRT) or by treating focally the nodal relapse using Stereotactic Body Radiotherapy (SBRT) with three different strategies (involved-node, involved-site, and involved-field) (; ).
Figure 1. Color wash representation (95% of the prescribed dose) of the different radiotherapy treatment strategies for nodal oligorecurrent prostate cancer patients. Involved node SBRT, 30 Gy in 3 fractions; Involved site SBRT, 24 Gy in 3 fractions with a simultaneous integrated boost (SIB) at 30 Gy; Involved field RT, 50.4 Gy in 28 fractions; ENRT, 45 Gy in 25 fractions with SIB of 65 Gy on PET-positive nodes; Super-extended ENRT: 45 Gy in 25 fractions with SIB of 65 Gy on PET-positive node.
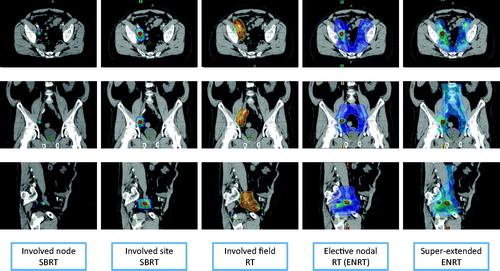
Table 1. Outcome and toxicity rates of the different RT strategies for nodal oligorecurrent prostate cancer patients.
The aim of this systematic review is to present the rationale and the results of the different RT strategies for nodal recurrence treatment and then to compare them in an effort to improve the management of nodal oligorecurrent hormone-sensitive PCa by defining the optimal target volume of salvage RT treatments.
Material and methods
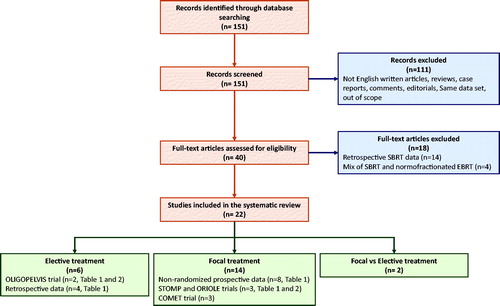
A systematic review of the literature was conducted on the Pubmed database according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [Citation35]. The following keywords were entered to identify articles: (“prostate” OR “prostate cancer”) AND (“oligometastases” OR “oligometastatic” OR “oligorecurrent” OR “node recurrences” OR “node recurrence” OR “nodal recurrences” OR “nodal recurrence) AND (“radiotherapy” OR “radiation” OR “irradiation”) AND (“node” OR “nodal” OR “nodes” OR “lesions” OR “lesion”) AND (“stereotactic” OR “elective” OR “pelvis” or “pelvic” or “guided”). 151 articles published before May 17th, 2020 were identified and screened. 111 were excluded because being irrelevant, reviews, case reports, not written in English, comments or editorials, or articles using the same dataset of other articles. We then excluded papers on focal treatment with retrospective analysis and papers with both focal and elective treatment with no clear distinction. At the end, 22 articles were included in the present review. The PRISMA flowchart is presented in .
Results
Focal treatment
Rationale
The number of publications of metastasis-directed therapy (MDT) in the field of bone or nodes recurrent PCa is rapidly increasing in step with growth in publications regarding 68Ga-PSMA PET-CT [Citation36]. The majorities of these series are retrospective, based on single institution experiences and a small number of patients, and converge toward the feasibility of a single course or repeated SBRT treatments. Excellent local control (LC) rates and very low acute and late toxicity events have been reported. Questionable oncological primary endpoints like ADT- or progression-free survivals (PFS) have been used in these studies, with no overall survival (OS) benefit demonstrated yet. However, by postponing ADT and the potential adverse effects of androgen ablation [Citation37], MDT strategies can potentially be used to preserve or improve the overall quality of life (QoL) in this category of patients, even if this goal remains hypothetical so far. It is also worth mentioning that this therapeutic strategy would not generate additional expenses to the health care system. Based on a recent cost-utility study using a Markov-model, MDT seems to be potentially cost-effective in the treatment of oligorecurrent PCa patients compared to immediate or delayed ADT [Citation38].
ADT-free survival and PFS after focal MDT
Several retrospective studies have evaluated the role of MDT for nodal oligorecurrent PCa patients [Citation27,Citation39–47], with some of them reporting ADT-free survival rates [Citation39,Citation42–45] ranging between 15.6 months [Citation45] and 44 months [Citation42].
Prospective non-randomized SBRT data are limited [Citation23–26,Citation28,Citation48]. Amongst them the Australian POPSTAR trial notably reported in 22 castration sensitive patients restaged with NaF PSMA and treated with a 20 Gy single dose SBRT a promising 2-year ADT-free survival rate of 48% [Citation23] while others studies [Citation25,Citation48] reported results of repeated SBRT sessions (the so called ‘Pokemet strategy’ [Citation36]).
Only two randomized phase II trials evaluated prospectively the role of MDT compared to observation in the PCa oligorecurrent setting [Citation20,Citation22] (). In the Belgian Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP) trial [Citation22], the median ADT-free survival rate improved from 13 months in the observation group to 21 months in the MDT arm (surgery or SBRT) (HR 0.60, 80% CI, 0.40 to 0.90). Similarly, biochemical relapse-free survival was also improved with MDT (HR 0.52, 80% CI, 0.36 to 0.76). MDT was well tolerated, with no severe treatment-related toxicity and no detrimental impact on health-related QoL. In the Baltimore Observation versus stereotactic ablative Radiation for OLigometastatic prostate CancEr (ORIOLE) trial [Citation20], there was a clear PFS benefit from ablative treatment of all PSMA avid lesions compared to the surveillance arm (Hazard Ratio 0.3, 95% Confidence Interval, 0.11 to 0.81).
Table 2. Published and ongoing Phase II clinical trials investigating different radiotherapy volumes in the setting of nodal oligorecurrence.
Finally, a randomized phase 2 open-label trial has compared standard of care palliative treatment with or without ablative SBRT in oligometastatic patients of different origin. This study showed an OS benefit by adding SBRT to systemic treatment. However, PCa patients represented only the 21% of the experimental, limiting reliable conclusions concerning the role of ablative SBRT in this subgroup of patients [Citation50].
Treatment volumes and RT techniques
Involved node SBRT
Volumes
The treatment volume includes the single node (gross tumor volume, GTV) as defined on restaging imaging and a planning target volume (PTV) isotropic margin of 1 to 8 mm depending on the IGRT technique used, bypassing the delineation of the clinical target volume (CTV).
Radiation dose and fractionation
Different RT regimens have been proposed to irradiate nodal recurrences with an involved node SBRT in retrospective and prospective studies. The SBRT schedules used for involved node SBRT ranges from single doses of 20–24 Gy to more fractionated schedules up to 5 fractions, with a total biologically effective dose ranging from 80 Gy to 270 Gy using a α/β ratio of 3 Gy [Citation23–28,Citation39,Citation41,Citation44–46,Citation48]. Excellent 2-year LC rates of 70% to 100% have been observed with these schedules, even if a direct comparison between them is limited by the different dose prescription [Citation48]. For example, Decaestecker et al. reported a 100% LC rate at 2 years with no grade 3 toxicity delivering 3 fractions of 10 Gy on alternate days, one of the most commonly used SBRT schedules [Citation25]. In this trial, the dose was prescribed to the periphery of the PTV with 80% of the dose covering 90% of the target volume. A multi-institutional analysis including 119 oligometastatic patients with 163 metastases revealed that higher dose was correlated with higher local control rates with a with a 3-yr local PFS of 79% for patients treated with a biologically effective dose ≤100Gy versus 99% for patients treated with >100Gy (p = .01) [Citation47].
Toxicity
Studies evaluating involved node SBRT in the oligorecurrent setting converge toward the fact that these treatments are extremely well tolerated [Citation23–28,Citation39,Citation41,Citation44–46,Citation48]. Taken together, more than 300 patients have been treated with involved node SBRT with only one of them having developed late grade 3 toxicity, while others experienced no more than grade 2 acute and/or late toxicities. In the series from Ingrosso et al., one patient treated with 5 fractions of 7 Gy or 8 Gy developed a grade 3 retroperitoneal fibrosis 12 months after the end of SBRT, complicated with bowel obstruction that required a surgical treatment [Citation39].
Patterns of relapse and the ‘pokemet strategy’
Ost et al. retrospectively analyzed the patterns of failure after involved node SBRT [Citation42]. They demonstrated that the majority of patients relapse with a new oligometastatic status, suggesting that SBRT sessions could therefore be repeated until widespread progression, thus delaying the use of systemic therapy as it was found to be the case for a single SBRT session [Citation22]. This strategy has been tested in two single center prospective studies [Citation25,Citation48].
In the first study, Decaestecker et al. [Citation25] treated 50 oligometastatic PCa patients with SBRT, repeating a metabolic imaging in case of biochemical failure: if 3 or less metastases were detected, SBRT was performed again and if a polymetastatic status was found, ADT was initiated. After a median follow up of 2 years, 18 patients were disease-free and 32 experienced distant metastatic progression resulting in a median ADT-free survival of 25 months (95% CI: 20–30 months). For patients with initial pelvic lymph node metastases, 67% of the relapses were located in the pelvic or retroperitoneal nodes and 33% in the bones. For patients with initial bone metastases, 88% of the relapses continued to be observed in the bones. In this study, the majority of patients were staged with FDG PET-CT and only a minority with choline PET-CT, thus a proportion of patients might have been under-staged [Citation51].
Two years later, a study with the same design has been led by Pasqualetti et al. [Citation48] (with updated results published this year [Citation21]) using choline PET-CT to stage or restage oligometastatic PCa patients before and after SBRT. Twenty-nine patients with oligometastatic PCa were included and after a median follow up of 11.5 months, 20 patients did not receive any systemic therapy, resulting in a median ADT-free survival of 39.7 months (95% CI: 17.2–62.1 months). Six out of 10 patients with nodal recurrence experienced a relapse in close proximity to the previously treated nodes. Every oligorecurrent patient except one relapsed with the same pattern of the first diagnosed metastasis (bone–bone, node–node).
Involved site SBRT
In order to limit nodal relapses close to the PET-positive lymph-node, Kneebone et al. treated 57 oligorecurrent PCa patients using a two dose-level PTV volume around the involved node: one high-dose PTV (nodal GTV + 5mm) to 50 Gy in 5 fractions or 30 Gy in 3 fractions, and one low-dose PTV consisting of a 5 mm margin around a low-dose CTV generated by expanding the GTV with a 10 mm along the nodal chains and irradiated to lesser dose (30 Gy in 5 fractions or 24 Gy in 3 fractions) [Citation28]. After a median follow-up of 16 months, 43 patients (75%) experienced biochemical failure. Among the 37 patients with nodal disease, the majority (N = 23) relapsed again in the nodes, and only 3 relapsed in the bones. Details on the anatomical site of relapse site were not reported.
Involved field RT
In a 2018 study by Soldatov et al. irradiation of the nodal oligorecurrent disease was performed by treating the involved node with a CTV encompassing the lymph node drainage region up to the next vessel bifurcation without the whole ipsilateral lymphatic drainage [Citation29]. Using a 10-mm PTV margin, patients were treated with standard fractionation up to a dose of 50.4 or 60 Gy. After a median follow-up of 18 months, 43.5% (47 of 108) of the patients experienced a biochemical progression. On restaging imaging (68Ga-PSMA PET-CT), a metastatic spread was observed from the iliac region to the retroperitoneum and to the bones, with half of the patients presenting a polymetastatic relapse.
As compared to other focal treatments, larger fields used in involved field RT can decrease the overall biochemical relapse rate at 18 months (43.5% versus 75% for involved site SBRT in the Kneebone series [Citation28]). Moreover, relapses observed with involved field RT are mostly observed in the distant lymph node stations, on the contrary of the relapses in close proximity of the irradiated regions observed with an involved node strategy [Citation48]. Similarly, half of the patients treated with involved field RT relapse in a widespread manner compared to only a 5.4% of the patients treated with an involved node strategy [Citation52].
Elective treatment
Rationale
As molecular imaging may underestimate the nodal burden [Citation16,Citation53] and relapses after focal SBRT are mainly nodal [Citation42], a WPRT encompassing prophylactic nodal drainage regions might be a valid treatment alternative to focal strategies with the attempt to increase PFS. Demonstration that adjuvant WPRT can potentially eradicate micrometastases and improve PFS has been observed in a study by Rischke et al. [Citation54]. After sLND for pelvic oligorecurrent PCa, adjuvant WPRT improved significantly PFS compared to sLND alone (70.7% vs. 26.3%, respectively).
PFS after ENRT
The majority of data on ENRT are coming from retrospective series, with reported PFS rates at 3-years ranging between 58% and 75%. Tran et al., updating a previous series from Schick et al. [Citation55] reported in 53 oligorecurrent patients (<5 nodes: N1 or M1a) treated with ENRT (45 or 50 Gy prophylactically with a boost to the positive LNs with a median dose of 64.4 Gy) a promising 5-year biochemical disease-free survival and distant PFS of 43% and 58%, respectively [Citation31]. Similarly, in a smaller German series from Wurschmidt et al. the 3-year biochemical disease-free survival and distant PFS in19 patients treated with 45–50 Gy by boosting the PET-positive nodes up to 66 Gy was 49% and 75%, respectively [Citation56].
Fodor et al. reported a 3-year OS, local and clinical relapse-free survival of 80%, 89.8% and 61.8%, respectively for 72 patients treated with choline PET-guided ENRT to the pelvic and/or lombo-aortic region (median dose 51.8 Gy) with a simultaneous integrated boost (SIB) up to 65.5 Gy to the positive nodes [Citation32].
The Oligopelvis – GETUG P07 is the only prospective trial [Citation57] ( and ) evaluating in oligorecurrent PCa patients with ≤5 pelvic nodes the role of pelvic intensity-modulated radiation therapy with 6 months of concomitant ADT in terms of tolerance, outcome and QoL impact. While acute toxicity data have been recently published [Citation49], PFS results, the primary endpoint of the study, have been presented during the 2020 ASCO GU meeting [Citation30,Citation57]. After a median follow-up of 34 months, the 2- and 3-year PFS rates was 77.6% and 52.4%, respectively.
Treatment volumes and RT techniques
Volume and patterns of relapse
WPRT
Definition of the CTV follows the international guidelines for WPRT using the Radiation Therapy Oncology Group (RTOG) contouring guidelines [Citation58], including the distal common iliac, external iliac, internal iliac, pre-sacral, obturator nodal regions. The upper CTV level ranges from the L5-S1 interspace to the L4-5 level at the aortic bifurcation.
Contouring guidelines defined within the PIVOTAL trial are also used [Citation59], with differences mainly observed in the upper limit (lower border of L5 vertebra) of treatment field and the coverage of the pre-sacral region.
Super-extended ENRT
The mapping of nodal relapses with PET-CT following standard curative treatments revealed that the above pelvis volumes may be insufficient to cover all disease sites.
De Bruycker et al. mapped all nodal recurrences of 82 patients with biochemical recurrence following a curative treatment using choline PET-CT (≤5 nodes; N1/M1a) [Citation60]. Forty-nine presented recurrences in the true pelvis, followed by the common iliac nodes (10%), the retroperitoneal/distant inguinal (10%), and a combination of these (31%). Using an adapted version of the PIVOTAL template [Citation59], including the common iliac nodes up to the aortic bifurcation (top of L4), a coverage of the 80% of all pelvic lesions can be expected. Possible improvement in these rates could be expected by extending the template more distally up to the mid-femoral head.
In a similar analysis from Calais et al. using a PSMA PET-CT imaging in 270 recurrent PCa patients, a WPRT CTV definition using the RTOG guidelines resulted to miss the nodal relapses in 39% of the patients [Citation3]. The majority of the patients relapsed in the bones (23/52, 44%) and perirectal LNs (16/52, 31%). The RTOG template would have missed 24% of true pelvic nodes, a rate which is similar to the 20% observed by De Bruycker et al. [Citation60].
Finally, Lepinoy et al. have implemented this salvage super-extended ENRT in 27 nodal oligorecurrent patients. For these patients, the upper limit of the standard RTOG pelvic CTV volume was extended up to the L2–L3 interspace when the common iliac or lower para-aortic stations were involved, and up to the renal arteries in case of positive nodes in the lombo-aortic region [Citation33]. Salvage super-extended ENRT was overall well tolerated with no acute and late toxicity higher than grade 2. Similar results with salvage ENRT were observed by Jethwa et al. in a cohort of oligorecurrent patients treated in 90% of the cases with treatment fields extending above the standard L5-S1 RTOG pelvic limit [Citation34].
Radiation dose and fractionation
In the different ENRT retrospective series [Citation31,Citation32,Citation55,Citation56], the median prophylactic dose to the bilateral nodal drainage regions ranged between 45 and 51.8 Gy, with a dose escalation up to 65–66.6 Gy on the PET-positive nodes, mostly using a SIB technique.
Namely, in the Oligopelvis – GETUG P07 trial the prescribed dose was 54 Gy to the whole pelvis (30 fractions of 1.8 Gy) with a 66 Gy SIB on choline PET-positive nodes (30 fractions of 2.2 Gy). The prostate bed received 66 Gy (if untreated) with a 72 Gy boost to the possible local relapse [Citation57].
Toxicity
By irradiating large treatment volumes, the mostly expected toxicity from ENRT remains gastro-intestinal (GI) [Citation61,Citation62] with mainly acute GI events reported in the different series [Citation32,Citation56,Citation63,Citation64]. However, respect of dose constraints by lowering the bowel volumes receiving more than 40–50 Gy thanks to modern ENRT techniques can reduce further the risk of acute side effects [Citation65]. Few series reported on long-term toxicities, with higher rates for both genito-urinary (GU) and GI toxicity if compared to SBRT treatment [Citation63,Citation66].
In the Oligopelvis – GETUG P07 trial, the grade 2 and 3 urinary acute toxicity rates for the 67 enrolled patients were 13.4% and 4.4%, respectively, while grade 2 acute GI toxicity rate was 14.9%. Patient’s QoL as measured by European Organization for Research and Treatment of Cancer (EORTC) questionnaires remained stable at 1 year of follow-up for the urinary, bowel and ADT related domains. As expected, sexual activity decreased at 6 months in relation to ADT blockage [Citation49].
Involved node SBRT versus ENRT
Comparative data assessing results of SBRT or ENRT approaches are scarce in literature.
Lepinoy et al. retrospectively compared toxicities and time to failure in 62 nodal oligorecurrent PCa patients treated with involved node SBRT or super-extended ENRT [Citation33]. After a median follow up of 41.8 months, no differences in acute and late toxicity were observed between the two treatment techniques. On the other hand, outcome was significantly improved with ENRT, with an 88.3% PFS rate at 3 years compared to a 55.3% observed in the SBRT cohort [Citation33].
In a larger retrospective series of 506 nodal oligorecurrent PCa patients with 5 or less positive pelvic nodes, the outcome and toxicity results of a SBRT (n = 309) or ENRT (n = 197) technique were compared [Citation66]. Although ENRT was able to improve pelvic PFS compared to SBRT, the 3-year metastasis-free survival (appearance of any M1a, b and/or c recurrence) was similar between the two groups for patients with 2 to 5 nodal lesions. On the other hand, MFS was improved with ENRT for patients with a single recurrent node (HR: 0.50, 95% CI 0.3–0.85, p = .009), at the price of an overall higher rate of late toxicities (16% for ENRT vs 5% for SBRT, p < .01).
The ongoing randomized, phase II, prospective PEACE V-STORM trial () will provide new insights on the best treatment approach for PCa patients with a nodal oligorecurrent disease [Citation67]. With extra-pelvic metastasis-free survival as primary endpoint, the study will randomize patients between a MDT strategy (sLND or SBRT) versus a MDT treatment completed by a WPRT (45 Gy in 25 fractions after sLND or the same fractionation with a SIB boost to the positive nodes). In both arms of the trial use of 6 months of concomitant ADT is mandatory.
Discussion and conclusions
With the widespread use of molecular imaging, a large proportion of recurrent PCa patients are diagnosed with an oligorecurrent disease, a status amenable to a curative treatment and characterized by an overall good prognosis [Citation68]. More than half of these patients present a disease localized to the pelvis [Citation2,Citation69,Citation70]. The best treatment approach of this specific group of patients remains therefore of crucial importance.
Immediate or differed ADT remains the level I evidence-based treatment for these patients, with observation recommended in selected frail and aged patients [Citation5,Citation11]. However, MDT is widely gaining acceptance in this setting in an attempt to cure patients and/or delay the initiation of ADT. The Australian and New Zealand Radiation Oncology Genito-Urinary group recommend that RT may be as suitable treatment option as ADT, observation or surgery for recurrence limited to pelvic LNs [Citation71]. In the 2019 Advanced PCa Consensus Conference (APCCC), 75% of the panelists recommended systemic therapy and local treatment of all lesions for the majority of patients with oligorecurrent-oligometastatic PCa [Citation72].
SBRT and ENRT can be considered valid and effective MDT strategies for these patients (), both of them being able to improve ADT-free survival with acceptable toxicity rates. While ENRT offers better nodal coverage and improves PFS [Citation31,Citation32,Citation55,Citation56], SBRT can improve LC, delay ADT and limit toxicity and the impact on patient QoL [Citation52].
Table 3. Focal versus elective radiotherapy treatment strategies.
As for now, retrospective data comparing both irradiation techniques seem to favor ENRT over SBRT in terms of improved freedom from failure and fewer nodal recurrences, at the cost of a potential increased toxicity [Citation33,Citation66]. Moreover, the only experts’ consensus addressing the question of the RT target volume in this setting also supports the use of ENRT rather than SBRT given the high risk of subclinical disease beyond what is identified on PET imaging [Citation71]. However, the low level of evidence of experts’ consensus and retrospective analyses cannot guide us to perform ENRT rather than SBRT. Results from the PEACE V-STORM trial [Citation67] that will address this issue in a randomized prospective phase II study are therefore eagerly awaited.
Clinical goals of MDT strategies in the oligorecurrent setting have not so clearly defined, ranging from delaying use of palliative ADT, to postponing castration resistance and, for selected cases, to potentially eradicate and cure relapsing disease. If one of the stronger rationales for using RT alone is to delay the initiation of ADT and their related side effects, a synergistic effect may be expected by adding ADT to MDT strategies. Based on results of randomized trials of salvage RT [Citation73–75] showing an improvement in PFS and OS with addition of ADT, it seems realistic to translate a similar benefit to MDT strategies in case of oligorecurrent nodal disease. Nevertheless, despite retrospective series suggested better PFS rates with concomitant ADT [Citation47,Citation76], level I evidence on indications and duration of ADT combined with MDT strategies in the nodal oligorecurrent setting is still lacking. If prospective trials are combining MDT with 6 months of ADT [Citation57,Citation67], better biochemical relapse-free survival rates have been observed with a ADT duration of more than 6 months [Citation76]. Ongoing prospective randomized studies like the ADOPT trial (NCT040302454) randomizing between MDT with or without ADT will shed light on this issue.
As included in the last EAU guidelines [Citation5], sLND represents a valid treatment option for nodal oligorecurrent disease, with promising 2-year biochemical progression-free survival rates ranging from 23% to 64% [Citation77]. In analogy to the different salvage RT strategies, a large variability, from a limited to more extensive surgical templates, exists even for sLND; by limiting the nodal dissection to the true pelvis, more than half of the nodal recurrences are however missed [Citation60]. Therefore, based on the study from De Bruycker et al., to ensure coverage of the majority of the relapse sites and maximize treatment outcomes, use of superextended sLND or ENRT modalities should be the preferred option for patients with nodal oligorecurrent disease. On the other hand, the role of adjuvant WPRT after sLND remains an open issue actually investigated prospectively by the STORM-PEACE 5 trial [Citation67].
It is not excluded that the presence or absence of some predictive factors at recurrence like the PSA doubling time, a high-risk disease at diagnosis, and/or the number of nodal involved will help to guide the most adapted treatment strategy [Citation18]. Despite the existing heterogeneities in treating these patients, results of the De Bleser et al. series suggests an advantage in reducing nodal relapses with ENRT, especially for patients with a single nodal recurrence [Citation66]. Similarly, among 14 Swiss radiation oncology centers there was some consensus to recommend ENRT with concomitant ADT for fit patients with unfavorable tumor characteristics such as short PSA doubling time (<6 months) or initial high-risk disease [Citation18]. However, independently from the MDT strategies proposed, it should be noted that some oligorecurrent PCa patients present at relapse a sublinical extrapelvic metastatic disease that will rapidly evolve to a clinical polymetastatic status. Translational research and early identification of biomarkers including microRNA profiling will probably help in the next future to guide and personalize treatment for oliogorecurrent PCa [Citation78–80].
Finally, a lot of countries cannot afford performing molecular imaging for restaging in the biochemical recurrent setting. Systematic inclusion of the elective pelvic nodal regions in the RT volumes together with prostate bed irradiation should therefore remain the standard volumes for blinded salvage RT treatment post-prostatectomy as proposed by the RTOG 0534 SPORRT trial (NCT00567580).
Furthermore, continuous improvements in systemic therapies, such as addition of androgen receptors pathway inhibitors or docetaxel to standard LH-RH ADT therapies in the hormone-sensitive disease setting [Citation81–84], will certainly challenge the always evolving treatment landscape of oligorecurrent PCa.
In conclusion, the RT management of nodal oligorecurrent patients, a new clinical entity emerging with the use of novel imaging techniques, is a rapidly changing field which brought its share of unanswered questions. Large variability exists in RT target volumes for oligorecurrent nodal PCa, ranging from involved node SBRT to elective treatments with, between these extremes, the involved site and involved field strategies. Despite the fact that a more comprehensive pelvic nodal irradiation seems to improve PFS compared to focal SBRT series, a benefit in stronger outcome endpoints has not yet been demonstrated for larger treatment fields and the optimal RT strategy remains to be determined in the setting of nodal oligorecurrent PCa. Results from ongoing trials will hopefully help physicians to guide the management of these patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tilki D, Mandel P, Schlomm T, et al. External validation of the CAPRA-S score to predict biochemical recurrence, metastasis and mortality after radical prostatectomy in a European cohort. J Urol. 2015;193:1970–1975.
- Parker WP, Davis BJ, Park SS, et al. Identification of site-specific recurrence following primary radiation therapy for prostate cancer using C-11 choline positron emission tomography/computed tomography: a nomogram for predicting extrapelvic disease. Eur Urol. 2017;71:340–348.
- Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237.
- Devos G, De Meerleer G, Joniau S. Have we entered the era of imaging before salvage treatment for recurrent prostate cancer? Eur Urol. 2019;76:265–267.
- Mottet N, Briers E, Cornford P, et al. Prostate cancer guidelines. 2019. Available from: https://uroweb.org/guideline/prostate-cancer/
- Ost P, Decaestecker K, Lambert B, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74:297–305.
- Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659.
- Ploussard G, Almeras C, Briganti A, et al. Management of node only recurrence after primary local treatment for prostate cancer: a systematic review of the literature. J Urol. 2015;194:983–988.
- Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67:852–863.
- Steuber T, Jilg C, Tennstedt P, et al. Standard of care versus metastases-directed therapy for PET-detected nodal oligorecurrent prostate cancer following multimodality treatment: a multi-institutional case-control study. Eur Urol Focus. 2019;5:1007–1013.
- NCCN Guidelines Prostate Cancer. 2019. Version 4. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Calais J, Ceci F, Eiber M, et al. (18)F-Fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294.
- Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937.
- Muller J, Ferraro DA, Muehlematter UJ, et al. Clinical impact of (68)Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur J Nucl Med Mol Imaging. 2019;46:889–900.
- Ferraro DA, Muehlematter UJ, Garcia Schuler HI, et al. (68)Ga-PSMA-11 PET has the potential to improve patient selection for extended pelvic lymph node dissection in intermediate to high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:147–159.
- Fossati N, Suardi N, Gandaglia G, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol. 2019;75:176–183.
- van Kalmthout LWM, van Melick HHE, Lavalaye J, et al. Prospective validation of gallium-68 PSMA-PET/CT in primary staging of prostate cancer patients. J Urol. 2019;203.
- Panje C, Zilli T, Pra AD, et al. Radiotherapy for pelvic nodal recurrences after radical prostatectomy: patient selection in clinical practice. Radiat Oncol. 2019;14:177.
- Lancia A, Zilli T, Achard V, et al. Oligometastatic prostate cancer: the game is afoot. Cancer Treat Rev. 2019;73:84–90.
- Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650.
- Pasqualetti F, Panichi M, Sollini M, et al. (18)F]Fluorocholine PET/CT-guided stereotactic body radiotherapy in patients with recurrent oligometastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:185–191.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453.
- Siva S, Bressel M, Murphy DG, et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74:455–462.
- Jereczek-Fossa BA, Fanetti G, Fodor C, et al. Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer. 2017;15:e623–e632.
- Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;9:135.
- Jereczek-Fossa BA, Beltramo G, Fariselli L, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:889–897.
- Casamassima F, Masi L, Menichelli C, et al. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori. 2011;97:49–55.
- Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1:531–537.
- Soldatov A, von Klot CAJ, Walacides D, et al. Patterns of progression after 68Ga-PSMA-ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2019;103:95–104.
- Supiot S, Pasquier D, Buthaud X, et al. Oligopelvis-GETUG P07: a multicenter phase II trial of combined salvage radiotherapy and hormone therapy in oligorecurrent pelvic node relapses of prostate cancer. J Clin Oncol. 2020;38:93–93.
- Tran S, Jorcano S, Falco T, et al. Oligorecurrent nodal prostate cancer: long-term results of an elective nodal irradiation approach. Am J Clin Oncol. 2018;41:960–962.
- Fodor A, Berardi G, Fiorino C, et al. Toxicity and efficacy of salvage carbon 11-choline positron emission tomography/computed tomography-guided radiation therapy in patients with lymph node recurrence of prostate cancer. BJU Int. 2017;119:406–413.
- Lepinoy A, Silva YE, Martin E, et al. Salvage extended field or involved field nodal irradiation in 18F-fluorocholine PET/CT oligorecurrent nodal failures from prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:40–48.
- Jethwa KR, Hellekson CD, Evans JD, et al. 11C-Choline PET guided salvage radiation therapy for isolated pelvic and paraortic nodal recurrence of prostate cancer after radical prostatectomy: rationale and early genitourinary or gastrointestinal toxicities. Adv Radiat Oncol. 2019;4:659–667.
- Moher D, Shamseer L, Clarke M, et al.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- Murphy DG, Sweeney CJ, Tombal B. “Gotta Catch 'em All”, or do we? Pokemet approach to metastatic prostate cancer. Eur Urol. 2017;72:1–3.
- Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836.
- De Bleser E, Willems R, Decaestecker K, et al. A trial-based cost-utility analysis of metastasis-directed therapy for oligorecurrent prostate cancer. Cancers (Basel). 2020;12:132.
- Ingrosso G, Trippa F, Maranzano E, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–49.
- Lepinoy A, Cochet A, Cueff A, et al. Pattern of occult nodal relapse diagnosed with (18)F-fluoro-choline PET/CT in prostate cancer patients with biochemical failure after prostate-only radiotherapy. Radiother Oncol. 2014;111:120–125.
- Muldermans JL, Romak LB, Kwon ED, et al. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:696–702.
- Ost P, Jereczek-Fossa BA, Van As N, et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol). 2016;28:e115–20.
- Ponti E, Ingrosso G, Carosi A, et al. Salvage stereotactic body radiotherapy for patients with prostate cancer with isolated lymph node metastasis: a single-center experience. Clin Genitourin Cancer. 2015;13:e279–e284.
- Triggiani L, Alongi F, Buglione M, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116:1520–1525.
- Bouman-Wammes EW, van Dodewaard-De Jong JM, Dahele M, et al. Benefits of using stereotactic body radiotherapy in patients with metachronous oligometastases of hormone-sensitive prostate cancer detected by [18F]fluoromethylcholine PET/CT. Clin Genitourin Cancer. 2017;15:e773–e782.
- Detti B, Bonomo P, Masi L, et al. Stereotactic radiotherapy for isolated nodal recurrence of prostate cancer. World J Urol. 2015;33:1197–1203.
- Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12.
- Pasqualetti F, Panichi M, Sainato A, et al. [(18)F]Choline PET/CT and stereotactic body radiotherapy on treatment decision making of oligometastatic prostate cancer patients: preliminary results. Radiat Oncol. 2016;11:9.
- Vaugier L, Palpacuer C, Rio E, et al. Early toxicity of a phase 2 trial of combined salvage radiation therapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer (OLIGOPELVIS GETUG P07). Int J Radiat Oncol Biol Phys. 2019;103:1061–1067.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058.
- Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89.
- Vilela RA, Navarro NF, Faria ET, et al. Use of stereotactic body radiation therapy for oligometastatic recurrent prostate cancer: a systematic review. J Med Imaging Radiat Oncol. 2018;62:692–706.
- Budaus L, Leyh-Bannurah SR, Salomon G, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–396.
- Rischke HC, Schultze-Seemann W, Wieser G, et al. Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther Onkol. 2015;191:310–320.
- Schick U, Jorcano S, Nouet P, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013;52:1622–1628.
- Wurschmidt F, Petersen C, Wahl A, et al. [18F]Fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44.
- Supiot S, Rio E, Pacteau V, et al. OLIGOPELVIS – GETUG P07: a multicentre phase II trial of combined salvage radiotherapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer. BMC Cancer. 2015;15:646.
- Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387.
- Harris VA, Staffurth J, Naismith O, et al.; PIVOTAL Trialists. Consensus guidelines and contouring atlas for pelvic node delineation in prostate and pelvic node intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:874–883.
- De Bruycker A, De Bleser E, Decaestecker K, et al. Nodal oligorecurrent prostate cancer: anatomic pattern of possible treatment failure in relation to elective surgical and radiotherapy treatment templates. Eur Urol. 2019;75:826–833.
- Aizer AA, Yu JB, McKeon AM, et al. Whole pelvic radiotherapy versus prostate only radiotherapy in the management of locally advanced or aggressive prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1344–1349.
- Tharmalingam H, Choudhury A, Van Herk M, et al. New approaches for effective and safe pelvic radiotherapy in high-risk prostate cancer. Nat Rev Urol. 2019;16:523–538.
- Franzese C, Fogliata A, D'Agostino GR, et al. Moderate hypofractionated radiotherapy with volumetric modulated arc therapy and simultaneous integrated boost for pelvic irradiation in prostate cancer. J Cancer Res Clin Oncol. 2017;143:1301–1309.
- Jorgo K, Polgar C, Major T, et al. Acute and late toxicity after moderate hypofractionation with simultaneous integrated boost (SIB) radiation therapy for prostate cancer. a single institution, prospective study. Pathol Oncol Res. 2019.
- Sini C, Noris Chiorda B, Gabriele P, et al. Patient-reported intestinal toxicity from whole pelvis intensity-modulated radiotherapy: first quantification of bowel dose-volume effects. Radiother Oncol. 2017;124:296–301.
- De Bleser E, Jereczek-Fossa BA, Pasquier D, et al. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: a multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol. 2019;76:732–739.
- De Bruycker A, Spiessens A, Dirix P, et al. PEACE V – salvage treatment of oligorecurrent nodal prostate cancer metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer. 2020;20:406.
- Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10.
- De Bruycker A, Lambert B, Claeys T, et al. Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int. 2017;120:815–821.
- Graziani T, Ceci F, Castellucci P, et al. C-Choline PET/CT for restaging prostate cancer. Results from 4,426 scans in a single-centre patient series. Eur J Nucl Med Mol Imaging. 2016;43:1971–1979.
- Lieng H, Hayden AJ, Christie DRH, et al. Radiotherapy for recurrent prostate cancer: 2018 recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother Oncol. 2018;129:377–386.
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020;77:508–547.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–756.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–428.
- Pollack A, Balogh AG, Low D, et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiation therapy: the NRG Oncology/RTOG 0534 SPPORT trial. ASTRO. 2018;102:1605.
- Kroeze SGC, Henkenberens C, Schmidt-Hegemann NS, et al. Prostate-specific membrane antigen positron emission tomography-detected oligorecurrent prostate cancer treated with metastases-directed radiotherapy: role of addition and duration of androgen deprivation. Eur Urol Focus. 2019.
- Ploussard G, Gandaglia G, Borgmann H, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: a systematic review. Eur Urol. 2019;76:493–504.
- Cheng HH, Mitchell PS, Kroh EM, et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One. 2013;8:e69239.
- Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat Oncol. 2014;9:230.
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One. 2011;6:e28650.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992–2003.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700.