Abstract
Background
Several studies show that subventricular zone (SVZ) contact of glioblastoma at diagnosis is a negative prognosticator of survival. In this report, we study glioblastoma patient survival, molecular biological and MRI-based volumetric findings according to SVZ contact.
Patients and methods
We conducted a retrospective study of adult patients diagnosed with supratentorial glioblastoma and uniformly treated with temozolomide-based chemoradiotherapy after surgery. The patient cohort was dichotomized according to tumor contact with the SVZ at diagnosis as determined on preoperative MR imaging. Tumor volume was measured using semi-automated segmentation technique. MGMT-gene promoter methylation and IDH mutation status were determined on stored tumor tissue. Kaplan-Meier survival curves were constructed. Cox regression analysis was used to adjust for known confounding factors of glioblastoma patient survival.
Results
A total of 214 patients were included in the study of whom 68% belonged to the SVZpos group. Median tumor volume was significantly larger in the SVZpos group (33,8 mL vs 15,6 mL; p < .001). MGMT-unmethylated glioblastoma was more frequent in the SVZpos group (61.4% vs 44.9%; p = .028). The overall survival and progression-free survival were 12.2 months and 5.9 months for the SVZpos patient group but 16.9 months and 10.3 months for the SVZneg group (log-rank p = .016 and .007 respectively). In multivariate Cox survival analysis, SVZ contact proved a negative prognostic parameter, independent from age, KPS, extent of resection, MGMT-methylation and IDH mutation status.
Conclusions
This study confirms SVZ contact at diagnosis as an independent negative prognostic factor for glioblastoma patient survival. SVZpos glioblastoma had larger tumor size and a larger proportion of unmethylated tumors than SVZneg glioblastoma. Further research is needed to establish whether the observed differences are solely explained by a different molecular profile of SVZpos glioblastoma or by interaction of glioblastoma with the unique SVZ microenvironment.
Introduction
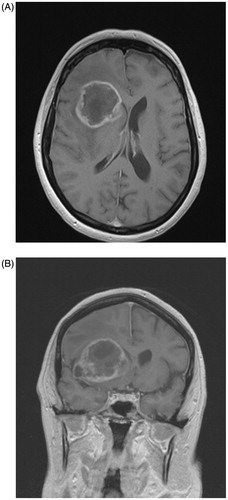
Glioblastoma is both the most frequent and most malignant primary brain tumor in adults. Almost all patients succumb to the disease, as reflected in a five-year survival rate of only 4.6% [Citation1]. This tumor typically presents on MR imaging of the brain as an irregularly ring-shaped zone of contrast-enhancement with central necrosis and surrounded by edema. If the contrast-enhancing part of the tumor abuts the subventricular zone (SVZ) at diagnosis, the tumor is considered as SVZ-contacting glioblastoma (SVZpos; ). In recent years, the SVZ has regained attention as the potential source of brain tumor initiating cells (BTICs), a hypothesis already formulated in 1944 by Globus and Kuhlenbeck [Citation2]. The evidence supporting the presence of BTICs in the SVZ is now rapidly growing and these cells probably originate from neural progenitor cells (NPCs) [Citation3–5]. Several retrospective studies acknowledge SVZ contact at diagnosis as a negative prognostic factor in glioblastoma [Citation6–9]. The negative influence of SVZ contact of glioblastoma seems to be independent from known prognostic factors such as age, Karnofsky Performance Score (KPS), extent of resection and molecular biological factors, specifically methylation of the 06-methylguanine-DNA-methyltransferase (MGMT)-promoter and isocitrate dehydrogenase (IDH)-mutation status [Citation7–9]. In this report, we conducted a retrospective study of a cohort of uniformly treated de novo glioblastoma patients with regard to survival and demographic, MRI-based volumetric and molecular biological differences between SVZ contact groups. The prognostic significance of SVZ contact was tested both in univariate and multivariate survival analysis, adjusting for independently validated prognosticators of glioblastoma patient survival [Citation10].
Patients & methods
Patient selection
We performed a retrospective analysis of adult (18 years or older) patients treated for supratentorial glioblastoma in two hospitals in Flanders (Belgium) between 2003 and 2014. Patients were included only if they completed temozolomide-based chemoradiotherapy (60 Gy in 30 fractions) after surgery. Patients with a previous history of low-grade glioma or other brain tumors were excluded. If the preoperative imaging could not be retrieved, these patients were also excluded.
The following demographic parameters were collected from the written or electronic medical files: sex; age at diagnosis; Karnofsky performance score (KPS). Surgery was classified into biopsy only or resection, based on surgical intent. Resection was classified into subtotal resection (STR) or gross total resection (GTR) based on postoperative imaging, according to the method applied by Stummer et al. [Citation11]. Briefly, if postoperative imaging showed the presence of contrast-enhancement of the size of one voxel or more, then surgery was classified as STR; if not, GTR was accepted. Overall survival (OS) was determined as the time between the date of histological diagnosis and the date of death. Progression-free survival (PFS) endpoint was determined by either the date of radiologic evidence of disease recurrence or progression or the date of change in treatment plan due to clinical disease progression. Patients who were still alive at the time of analysis were censored for OS. Patients without disease progression were censored for PFS at the time of the last registered follow-up visit.
This study was approved by the Ethics Committee of both participating hospitals (Belgian Registration number B670201730765; UZG 2016/1594; AZD 17004). Since the vast majority of patients had deceased at the time of analysis, the need for individual informed consent was waived by both committees.
Imaging and molecular biological factors
Contact of the contrast-enhancing part of the tumor with the SVZ was evaluated on preoperative MR imaging (SVZpos vs SVZneg). Preoperative tumor volume was measured on 3 D-T1 magnetization-prepared rapid acquisition gradient echo (MPRAGE) images with a slice thickness of 0.9 mm obtained for neuronavigation and using semi-automated segmentation technique [Citation12]. These images were acquired on 1,5 T or 3 T magnetic resonance imaging systems (Siemens, Erlangen, Germany).
The neuropathologist selected a representative formalin-fixed paraffin-embedded tissue block from the tumor tissue archive for each case. All samples were reviewed and tested for IDH-1 and −2 mutation using next-generation sequencing techniques. MGMT promoter methylation was determined using semi-quantitative methylation-specific polymerase chain reaction (qMSP), as previously described [Citation13].
Statistical analysis
Fisher's exact test was used to compare proportions between independent categorical variables of SVZpos and SVZneg groups and the independent samples t-test was applied for numerical variables, except for preoperative tumor volume. Mean difference in preoperative tumor volume between groups was assessed using a linear regression model, after log-transformation to improve normality of the measured tumor volumes. Kaplan-Meier survival curves for OS and PFS were plotted and compared between groups with the log-rank test; hazard ratios were also calculated using a univariate cox regression model. Next, a multivariate Cox regression model was fitted for survival analysis (OS and PFS), including known prognostic factors of survival in glioblastoma patients (age at diagnosis; KPS; biopsy vs resection; MGMT- methylation; IDH-mutation). In Cox regression models, numerical variables were not categorized [Citation14]. Graphical methods were used to assess that the proportional hazards assumption was respected in Cox models for categorical variables. For numeric variables, a time-dependent covariable was introduced in the model and checked that it was not significant. All statistical analyses were performed using SPPS (v26, IBM, Armonk, NY, USA). Statistical significance was set at p < .05 using two-tailed tests.
Results
In total, 399 patients were surgically treated for glioblastoma. Three patients were excluded because of cerebellar localization or the presence of other tumors; 43 were lost to follow-up; 93 patients were treated with shortened radiotherapy schedules or without temozolomide; diagnostic MR imaging could not be retrieved in 46 patients. So, 214 patients were included in the study of whom 12 were still alive at the time of database closure. The majority of patients (68%) belonged to the SVZpos group (). IDH mutation could not be determined due to technical reasons in 19.5% of patients while MGMT-methylation status is lacking in 8% of patients. Only the IDH1R132 mutation was found. The difference in tumor size and in MGMT-methylation status between SVZ contact groups proved statistically significant (). The number of unmethylated tumors was higher in the SVZpos group while in the SVZneg group the numbers of methylated and unmethylated tumors were proportionally distributed (44.9% unmethylated and 46.4% methylated; ). There was no difference in frequency of IDH-mutation between SVZ contact groups. Tumor volume could be determined in 177 patients. Median tumor volume was more than double in the SVZpos glioblastoma group (33.8 mL for SVZpos vs 15.6 mL for SVZneg; p < .001). There were no significant differences in age, KPS, female/male ratio or extent of surgical resection between SVZ contact groups.
Table 1. Overview of demographic, surgical, molecular biological and volumetric characteristics as well as survival according to subventricular zone contact group.
Univariate survival analysis showed that age, KPS, extent of resection, MGMT-methylation status and IDH-mutation correlated significantly with glioblastoma patient survival albeit IDH-mutation only for PFS (). Preoperative tumor volume did not correlate with survival in univariate Cox regression model. SVZ contact was a significant prognostic factor for both OS (SVZpos median OS 12.2 months vs SVZneg median OS 16.9 months; p = .016) and PFS (SVZpos median PFS 5.9 vs SVZneg median PFS 10.3 months; p = .007). After adjustment for age, KPS, surgical resection, IDH-mutation and MGMT-methylation status, SVZ contact proved a statistically significant prognostic factor both for OS and PFS (, ).
Figure 2. Kaplan-Meier curves of overall survival (A) and progression-free survival (B) in glioblastoma with (SVZpos) and without (SVZneg) subventricular zone contact.
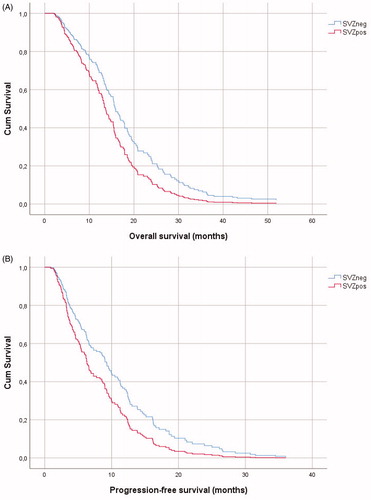
Table 2. Univariate survival analysis for several prognostic factors of glioblastoma patient survival.
Table 3. Multivariate Cox regression model of glioblastoma patient survival adjusted for age, KPS, extent of resection, MGMT-methylation and SVZ contact.
Discussion
This study confirms SVZ contact as an independent negative prognostic factor in glioblastoma patient survival. Compared to SVZneg tumors, SVZpos glioblastoma has a double median tumor volume and comprises predominantly MGMT-unmethylated tumors. There were no significant differences between SVZ contact groups with regard to sex ratio, age, KPS, extent of resection or IDH-mutation.
Contrary to our results, a recent meta-analysis of 6 reports on MGMT-methylation status of SVZpos glioblastoma, showed no significant difference between SVZ contact groups concerning this important prognostic epigenetic factor [Citation15]. This was also the case in the prospective study on SVZ contacting glioblastoma by Van Dijken et al. [Citation7]. However, a 2018 report by Han et al. on predicting MGMT promoter methylation status based on preoperative MR imaging, showed similar results to this study, with a significantly higher number of unmethylated tumors in the SVZpos group compared to the SVZneg group (58.3% vs 36.4% resp.; p = .012) [Citation16]. Literature does not allow to draw a definitive conclusion at present whether SVZpos glioblastoma has a different MGMT-methylation pattern than SVZneg tumors.
Importantly, despite the obvious higher number of unmethylated tumors in the SVZpos group, SVZ contact was an independent prognostic factor in multivariate survival analysis. Our survival results are analogous to the results of the 2017 meta-analysis on SVZ contact and glioblastoma patient survival [Citation6] and to those more recently obtained by Van Dijken et al. [Citation7] and Mistry et al. [Citation9]. In their 2019 study, Mistry et al. showed that SVZpos glioblastoma was associated with decreased survival and also with post-treatment hydrocephalus and leptomeningeal dissemination. This negative influence on glioblastoma patient survival of SVZ contact was also independent from ventricular entry during neurosurgical resection [Citation9]. Moreover, in another report studying TCGA molecular data of glioblastoma, the same group could not find a distinct molecular biological profile of SVZpos glioblastoma [Citation17]. Another group came to the same findings [Citation8]. If the molecular signature of SVZpos tumors is not fundamentally different from SVZneg glioblastoma, possibly the interplay between glioblastoma cells and the complex and unique SVZ niche may hold the key to understanding how SVZ contact of glioblastoma influences patient survival [Citation18]. For example, it has been shown in a mouse model that glioma cells invading the SVZ become radioresistant by influence of SVZ chemokines [Citation19].
The current finding of significantly larger tumor size for SVZpos glioblastoma is consistent with previously published findings [Citation7,Citation8,Citation15,Citation20]. Nevertheless, this observation alone is insufficient to establish the SVZ origin of glioblastoma. A direct correlation between anatomical localization of glioblastoma and its origin is unlikely [Citation20]. Mathematical glioma growth models demonstrate that tumors originating at a distance from the SVZ have a high likelihood of reaching the SVZ before detection [Citation21,Citation22]. SVZpos glioblastoma may just be the consequence of a highly malignant and rapidly growing tumoral lesion within the limited confinements of the brain rather than the indication of pure SVZ origin [Citation22].
Most importantly, this study acknowledges SVZ contact as a negative prognostic factor for glioblastoma patient survival, independent from age, KPS, extent of resection, MGMT-methylation status and IDH mutation (, ). In other words, SVZpos glioblastoma is even more aggressive than SVZneg tumors. The SVZ niche in glioblastoma patients may become a therapeutic target in the future whether by radiating the SVZ or by targeting specific components of the SVZ microenvironment [Citation5,Citation18]. We join the call already made by Smith et al. in 2016 to increase translational and basic research on the SVZ and its role in glioblastoma [Citation23].
This study has several shortcomings of which selection bias due to the retrospective study design may be the most important. Furthermore, corticosteroid use at diagnosis was not included in the analysis nor were the different treatment modalities applied for disease recurrence or progression. Nevertheless, we present a large series of glioblastoma patients who were uniformly treated in first tier treatment. We were able to include MGMT-promoter methylation status and IDH-mutation in the study and adjust survival analysis for these and other well-known prognostic factors.
Conclusion
In this glioblastoma patient cohort, SVZ contact at diagnosis is a negative and independent prognostic factor. The SVZpos patient group had significantly more MGMT-unmethylated and larger tumors. More studies are needed to be able to draw a definitive conclusion whether differences in MGMT-methylation pattern exist between glioblastoma SVZ contact groups. The role of SVZ contact in glioblastoma needs to be examined further in larger patient groups in order to establish how SVZ contact influences patient survival. The SVZ emerges as a potential therapeutic target in glioblastoma treatment.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of both participating hospitals (Belgian Registration number B670201730765; AZD 17004; UZG 2016/1594).
Informed consent
The need for informed consent was waived by the ethical committees of both participating hospitals.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Giorgio Hallaert and Harry Pinson. The first draft of the manuscript was written by Giorgio Hallaert and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgement
We gratefully acknowledge the help of Prof. em. Dr. M. Mareel with the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cantrell JN, Waddle MR, Rotman M, et al. Progress toward long-term survivors of glioblastoma. Mayo Clin. Proc. 2019;94(7):1278–1286.
- Globus JH, Kuhlenbeck H. The subependymal cell plate (matrix) and its relationship to brain tumors of the ependymal type. J Neuropathol Exp Neurol. 1944;3(1):1–35.
- Smith AW, Mehta MP, Wernicke AG. Neural stem cells, the subventricular zone and radiotherapy: implications for treating glioblastoma. J Neurooncol. 2016;128(2):207–216.
- Lee JH, Lee JE, Kahng JY, et al. Human Glioblastoma Arises From Subventricular Zone Cells with Low-Level Driver Mutations. Nature. 2018;560(7717):243–247.
- Altmann C, Keller S, Schmidt M. The role of SVZ stem cells in glioblastoma. Cancers. 2019;11(4):448–423.
- Mistry AM, Hale AT, Chambless LB, et al. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol. 2017;131(1):125–133.
- van Dijken BRJ, van Laar PJ, Li C, et al. Ventricle contact is associated with lower survival and increased peritumoral perfusion in glioblastoma. Journal of Neurosurgery. 2019;131(3):717–723.
- Berendsen S, van Bodegraven E, Seute T, et al. Adverse prognosis of glioblastoma contacting the subventricular zone: biological correlates. Plos One. 2019;14(10):e0222717.
- Mistry AM, Kelly PD, Gallant J-N, et al. Comparative analysis of subventricular zone glioblastoma contact and ventricular entry during resection in predicting dissemination, hydrocephalus, and survival. Neurosurgery. 2019;85(5):E924–32.
- Gittleman H, Cioffi G, Chunduru P, et al. An independently validated nomogram for isocitrate dehydrogenase-wild-type glioblastoma patient survival. Neurooncol Adv. 2019;1(1):1–9.
- Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–574.
- Henker C, Kriesen T, Glass Ä, et al. Volumetric quantification of glioblastoma: experiences with different measurement techniques and impact on survival. J Neurooncol. 2017;135(2):391–312.
- Pinson H, Hallaert G, Van der Meulen J, et al. Weak MGMT gene promoter methylation confers a clinically significant survival benefit in patients with newly diagnosed glioblastoma: a retrospective cohort study. J Neurooncol. 2020;146(1):55–62.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141.
- Mistry AM. Clinical correlates of subventricular zone-contacting glioblastomas: a meta-analysis. J Neurosurg Sci. 2017;63(5):1–7.
- Han Y, Yan L-F, Wang X-B, et al. Structural and advanced imaging in predicting MGMT promoter methylation of primary glioblastoma: a region of interest based analysis. BMC Cancer. 2018;18(1):215–210.
- Mistry AM, Wooten DJ, Davis LT, et al. Ventricular-subventricular zone contact by glioblastoma is not associated with molecular signatures in bulk tumor data. Sci Rep. 2019;9(1):1842–1812.
- Bardella C, Al-Shammari AR, Soares L, et al. The role of inflammation in subventricular zone cancer. Prog Neurobiol. 2018;170:37–52.
- Goffart N, Lombard A, Lallemand F, et al. CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone. Neuro-Oncology. 2017;19(1):66–77.
- Han S, Li X, Qiu B, et al. Can lateral ventricle contact predict the ontogeny and prognosis of glioblastoma?. J Neurooncol. 2015;124(1):45–55.
- Woodward DE, Cook J, Tracqui P, et al. A mathematical model of glioma growth: the effect of extent of surgical resection. Cell Prolif. 1996;29(6):269–288.
- Bohman L-E, Swanson KR, Moore JL, et al. Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery. 2010;67(5):1319–1318.
- Smith AW, Parashar B, Wernicke AG. Subventricular zone-associated glioblastoma: a call for translational research to guide clinical decision making. Neurogenesis (Austin). 2016;3(1):e1225548.