Abstract
Background
Cancer patients increasingly seek second opinion (SO) consultations, but there is scarce empirical evidence to substantiate medical and psychological benefits for patients. This is the first study to examine patient- and oncologist-reported (1) motivations and expectations of patients to seek a SO, (2) the perceived medical outcome, and (3) psychological consequences of SOs over time (i.e. patients’ uncertainty and anxiety).
Material and methods
This multi-informant longitudinal cohort study (SO-COM) included consecutive cancer patients referred for a SO (N = 70; age 28–85), as well as their referring and consulting oncologists. Outcome measures were completed at three time points: Patients and referring oncologists reported motivations and expectations before the SO (T0), patients and consulting oncologists reported the medical outcome of the SO (i.e. discrepancy between first and second opinion) immediately following the SO (T1), and patients reported their uncertainty and anxiety at T0, T1, and two months following the SO (T2).
Results
Cancer patients most frequently reported wanting expert advice, exhausting all options, and/or needing more information as motivations for SOs. Referring oncologists rather accurately anticipated these motivations, except most did not recognize patients’ information needs. The vast majority of patients (90.0%) received a medical advice similar to the first opinion, although 65.7% had expected to receive a different opinion. Patients’ uncertainty (F = 6.82, p=.002; η2=.22), but not anxiety (F = 3.074, p=.055, η2=.11) was significantly reduced after the SO.
Conclusions
SOs can yield psychological benefits by reducing patients’ uncertainty, but the added medical value remains debatable. Referring oncologists may not be fully aware of their patients’ information needs. Patients should be better informed about goals and benefits of SOs to better manage their expectations. More cost-effective ways of optimally providing medically and psychologically valuable SOs need to be explored.
Introduction
Cancer patients increasingly seek second opinions (SOs) in oncology, but rates vary [Citation1,Citation2]. Requesting a SO may be appealing to patients to double-check their own oncologist’s opinion, or gather more information and exhaust all options [Citation1]. Yet, empirical evidence of cancer patients’ motivations to seek a SO is limited and mostly retrospective [Citation3–6]. Moreover, it remains unknown whether referring oncologists are fully aware of what motivates their patients to ask for a SO, potentially preventing them from addressing patients’ needs.
The medical and psychological benefits of SOs have been debated [Citation7–9]. Proponents emphasize that SOs can yield benefits such as better treatment [Citation10]. Critics argue that most SOs only confirm initial diagnoses/treatment plans, or even cause treatment delays which could be harmful [Citation11,Citation12]. Research substantiating this discussion is limited and highly variable in its assessment of medical outcomes. Estimates of how frequently SOs lead to a change in diagnosis and/or treatment range from 2–69% [Citation1,Citation2]. Reasons for this wide range found in previous studies remain speculative, but may be explained by varying definitions of what constitutes a major/minor discrepancy between first and second opinion, or by differences between study populations (e.g. discrepancies may be more common in patients with early rather than advanced cancer stage), or by settings (e.g. discrepancies may be more frequent when evaluating treatment options compared to diagnostic SOs).
Moreover, patients’ awareness of discrepancies between first and second opinions, and their perception of medical outcomes of a SO have never been assessed. Exploring such subjective perceptions is essential, as they may be powerful indicators of how patients are psychologically affected by SOs. There may be variable effects on patients’ psychological well-being, depending on their motivations/expectations and perceived medical outcome. For example, uncertainty and anxiety may be diminished if the initial opinion is confirmed or when promising (alternative) treatment options are offered [Citation8,Citation10,Citation12,Citation13]. However, SOs may be laden with contradicting information and thus induce confusion or doubts about the first opinion [Citation2,Citation14–16], leading to increased rather than decreased uncertainty and anxiety.
Overall, there is a dearth of empirical evidence about SOs. Prospective and longitudinal studies are needed to substantiate the discussion about their usefulness and investigate how to optimize these consultations. Therefore, we aimed (1) to describe and compare patient-reported and referring oncologist-reported motivations and expectations of seeking a SO; (2) to assess and compare patient-perceived and consulting oncologist-perceived discrepancies between the first and second opinion (i.e. medical outcome); and (3) to examine psychological consequences of seeking a SO over time (i.e. patients’ uncertainty and anxiety).
Material and methods
Study design
Data presented here are part of a larger prospective cohort study about SOs in oncology (SO-COM). The SO-COM study includes audio-recordings of SOs and self-report surveys from patients, referring oncologists (i.e. who provided the first opinion), and consulting oncologists (i.e. who provided the SO). Survey data were collected before (T0), immediately/in the week after (T1), and two months after the SO (T2, ). A 2-month time frame was chosen to examine whether perceptions shift over time, after most patients had returned to their referring oncologist.
Figure 1. Flow chart of participant inclusion and overview of the SO-COM study procedures (incl. survey measures used in the presented analyses). Note: *N = 77 includes every respondent who provided self-report and/or had their SO audio-recorded, of which N = 72 had provided self-report data and complete data was available from N = 70 patients at T0.
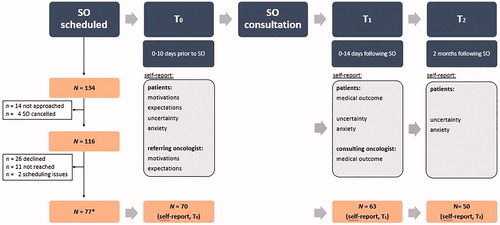
Consulting SO oncologists affiliated with two Dutch tertiary referral centers (Antoni van Leeuwenhoek hospital, AvL: a specialized adult oncology facility, and Utrecht University Medical Center, UMCU) were invited to participate and, if willing, signed informed consent forms. Consecutive patients (treated anywhere in the Netherlands) who were scheduled for a SO with participating oncologists, were informed about the SO-COM study by hospital staff and verbal consent was obtained to be contacted by the study team. Patients were then phoned for full study information and to obtain verbal consent for participation. Consenting patients were also asked for permission to contact their referring oncologist. Patients provided the name and affiliation of their oncologists who were subsequently approached by the research team.
Eligible participants were adult cancer patients with any type of solid tumor, scheduled for a SO, and sufficient Dutch language proficiency. Patients were mailed an information letter, informed consent form, and the first survey to be completed before the SO consultation (T0). The SO consultation was audio-recorded. Directly afterwards, the consulting oncologist completed a questionnaire and patients received the second survey (T1). Two months later, patients were mailed the third and final survey (T2; ). If patients had agreed, their referring oncologist was contacted. Referring oncologists who agreed, signed informed consent forms and completed one survey at T0 and a second survey two months later (T2). All procedures were approved by the institutional review board of the study sponsor/contractor (Amsterdam University Medical Center), as well as the local review boards of both participating hospitals; and all procedures are in accordance with the Helsinki Declaration. The STROBE cohort checklist was used when writing the present report [Citation17].
Measures
Patient factors
Patients provided sociodemographic information, including their age, sex, relationship status, and level of education. Medical personnel at both hospitals provided information about type of diagnosis, tumor staging and time since diagnosis ().
Table 1. Background characteristics of all patients (N = 70).
Motivations
Patients were provided with a checklist of eight potential reasons to seek a SO, based on previous research [Citation1]. They could add reasons as free text (). Referring oncologists were provided with the same list to assess their perceptions of patients’ motivations.
Table 2. Patient- and oncologist-reported motivations and expectations of seeking a SOa.
Expectations
Similarly to motivations, and based on the same previous research [Citation1], patients were provided with a list of nine possible expectations they may have of the SO and could add expectations as free text, if needed (). Referring oncologists completed a similar list to assess the extent to which they expected various outcomes of the SO (5-point Likert scale; completely disagree–completely agree).
Perceived medical consequences
Patients and consulting oncologists reported whether the medical outcome of the SO (e.g. diagnosis; advised treatment plan) was completely the same, mostly the same, mostly different, or completely different from the first opinion.
Psychological consequences
Patients’ uncertainty and anxiety were measured at all time points. Uncertainty was measured using the Mishel Uncertainty in Illness Scale (MUIS) [Citation18–20]. As done in previous research, 5 items relevant for this study’s context were used at T0 (i.e. focused on physicians’ information provision and patients’ uncertainty). Items were rated on a 5-point Likert scale (completely disagree–completely agree). The internal consistency of the scale was acceptable with a Cronbach’s alpha (α) of 0.65. Both at T1 and T2, a sixth MUIS-item was added assessing the perceived concordance of doctors’ opinions (α = 0.71 both times). Patients’ anxiety was measured using the 20-item ‘state’ scale of the Spiegelberger State-Trait Anxiety Inventory (STAI) [Citation21,Citation22], using a 4-point Likert-type response scale (not at all–very much). Internal consistency was excellent with α = 0.93–0.95.
Statistical analyses
Descriptive statistics were used to present patients’ motivations and expectations of seeking a SO (aim 1). Using χ2-tests, we tested whether the most common motivations and expectations differed by patients’ sociodemographic characteristics (i.e. sex, age, education), and whether they aligned with referring oncologists’ perceptions. For aim 2, we used descriptive statistics to present patients’ perceived medical outcome of the SO and compared it against consulting oncologists’ perceptions, using χ2-tests.
Potential changes in psychological outcomes over time were tested using repeated measures analyses of variance (ANOVA) for uncertainty and anxiety separately (aim 3). Depending on whether anxiety and/or uncertainty changed over time, additional repeated measures MANOVAs were planned, testing changes in either outcome while taking patients’ motivations, expectations, and perceived medical outcome into account. A-priori power calculations indicated a minimum required sample size of N = 42, based on repeated measures ANOVAs with 3 time-points and including between-within interactions (80% power, p=.05, effect size=.20; [Citation23] All analyses were carried out in IBM SPSS Statistics version 25.
Results
Recruitment
N = 134 eligible patients were identified, but due to logistical reasons, N = 116 patients were approached. Of these, 26 (22.4%) actively declined participation (e.g. due to feeling too sick/burdened by participation), 11 (9.5%) could not be reached, and two (1.7%) could not be included due to scheduling issues. Thus, N = 77 patients provided self-report data and/or had their SO audio-taped (response rate, RR = 66.4%; N = 77/116). Yet, complete self-report data at T0 were available from N = 70 patients and used in the present analyses (see also ). The completion rate from T0 to T1 was 90.0% (n = 63/70) and 71.4% at T2 (n = 50/70).
Participating patients were seen by 26 different consulting oncologists who all completed a survey at T1. Due to missing data, information on n = 53 out of 63 participating patients at T1 was used. The referring oncologists of 29 patients participated at T0 of whom 22 provided data used in the present analyses. Note that 57 patients had consented to their referring physicians being approached (RR = 50.9%; n = 29/57).
Patients
The included N = 70 cancer patients were aged 28–85 years at T0, predominantly female (n = 44, 62.9%), partnered/married (n = 63, 90.0%), and/or highly educated (n = 28, 40.0%). Most patients had been diagnosed with breast cancer (n = 22, 31.4%) or gastrointestinal tumors (n = 21, 30.0%), which constituted an advanced disease stage among the majority of patients (n = 61, 87.1%). Time since primary diagnosis ranged between 1 month to 31 years, because some patients presented with a new primary diagnosis and others with a relapse ().
Motivations
Most often, patients reported wanting to know what other specialists would advise (n = 46, 65.7%), wanting to be sure that they had tried everything (n = 42, 58.6%), wanting more information (n = 17, 24.3%), and/or that other people had advised them to seek a SO (n = 17, 24.3%; patients could indicate various motivations; see for the complete list). These top-4 motivations were not differently endorsed by different patients, except for ‘wanting to know what other specialists would advise’: Female patients (71.7% females vs. 28.3% males, χ2(1)=4.53, p=.033), younger patients (Mage=56.4 vs. 62.1, t(68)=2.25, p=.028), and/or highly educated patients (45.7% vs. vs. 34.8 (middle) vs. 19.6% (lower educated), χ2(2)=8.90, p=.012) were more likely to endorse this item. Moreover, highly educated patients more often endorsed ‘wanting more information’ (70.6% vs. 5.9% (middle) vs. 23.5% (lower educated patients), χ2(2)=9.74, p=.008).
Similar to patients, referring oncologists (n = 22) thought their patients were motivated by wanting to hear other specialists’ advise (n = 9, 40.9%) or making sure they had tried everything (n = 9, 40.9%; ). Given the low number of participating referring oncologists, we could not statistically test whether patient- and oncologist-report aligned.
Expectations
Patients most frequently expected to hear about different treatment options (n = 44, 62.9%), reassurance about the first opinion (n = 29, 41.4%), support in deciding between treatment options (n = 25, 35.7%; ), and/or obtain more information (n = 21, 30.0%). Endorsing those top-4 expectations did not differ by patients’ sex or age, but did by level of education: Highly educated people reported more often that they expected to receive help in choosing between treatment options (68.0% vs. 16% (middle) vs. 16.0% (low); χ2(2)=12.72, p=.002). In line with patients, referring oncologists also rated these four expectations as highest (). Overall, 65.7% of patients (n = 46/70) expected to receive a different opinion, including different treatment options, prognosis, and/or diagnosis ().
Perceived medical outcome
After the SO (T1), 56 of 63 participating patients reported that the medical outcome of their SO was ‘largely the same’ (n = 35, 55.6%) or ‘completely the same’ (n = 21, 33.3%) as the first opinion. Only 7 patients reported they received a ‘largely different’ (n = 5, 7.9%) or ‘completely different’ SO (n = 2, 3.2%; ). Of those 56 receiving a similar SO, 36 had expected to hear something different (64.3%), but did not. In line with patients, consulting oncologists reported in 92.4% of cases that their opinion was largely or completely similar to the first opinion (). Concordance between patient- and oncologist-perceived medical outcome could not be tested statistically due to empty cells, but only n = 2 larger discrepancies were observed (i.e. largely the same vs. entirely different).
Table 3. Descriptive statistics of patient- and oncologist-reported outcomes, and effects over time.
Psychological consequences
Baseline scores of uncertainty and anxiety did not differ by sex and were uncorrelated with age (p>.05). Repeated measures ANOVA indicated changes in levels of uncertainty over time (F = 6.82, p=.002; η2=.22; ). Specifically, uncertainty decreased from before (T0) to right after (T1) the SO (MΔ = 0.53, d = 0.63), and stayed relatively stable from T1 to T2 (MΔ=-0.08, d= −0.10, ). Accordingly, about half of patients (n = 32/63, 50.8%) showed a substantial decrease in uncertainty from T0-T1 (i.e. > 1/2SD), whereas only a small subset of patients (n = 8/63, 12.7%) reported a substantial increase in uncertainty (i.e. > 1/2SD) from T0-T1.
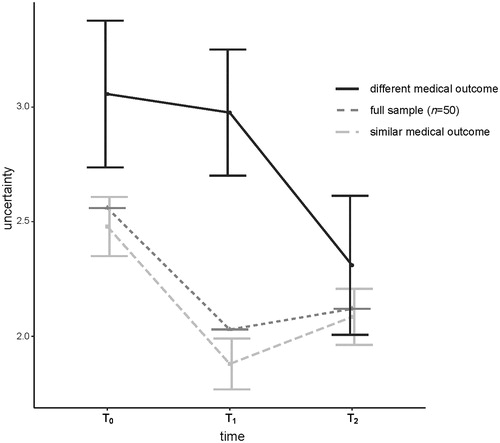
Figure 2. Estimated marginal means of uncertainty from T0-T2 for patients split by self-reported medical outcome (similar vs. different SO); and observed means for the full sample across three time points.
Changes in anxiety over time were borderline significant (F = 3.07, p=.055, η2=.11; ). Anxiety initially decreased somewhat from T0-T1 (MΔ = 0.14, d = 0.41), but scores increased again from T1-T2 back to almost baseline levels (MΔ= −0.07, d= −0.14). Among a quarter of patients (25.4%, n = 16/63) anxiety substantially decreased initially from T0-T1 (>1/2SD), whereas it substantially increased among 4 patients from T0-T1 (6.4%, n = 4/63).
Factors moderating changes in uncertainty
It was tested whether patients’ (a) motivations, (b) expectations, or (c) perceived medical outcome moderated the decrease in uncertainty, using repeated measures analyses. First, including the top-4 patient-reported motivations of requesting a SO (binary: yes/no), yielded no additional effects (all p > 0.5). Second, including patients’ expectations to receive a different treatment/prognosis/diagnosis (binary: yes/no) yielded no additional effect (p=.670). Finally, including the patient-reported medical outcome (binary: similar/different), indicated both the significant decrease of uncertainty over time and an interaction with medical outcome (F = 4.29, p=.019, η2=.15). Specifically, patients who perceived their medical outcome as largely/completely similar showed a steeper decline in uncertainty than the relatively small subset (n = 7) whose outcome was largely/completely different (). At T2, levels of uncertainty were almost equal among both groups.
Discussion
This prospective study is the first to examine patient- and oncologist-reported: (1) motivations and expectations for patients to seek a SO, (2) the perceived medical outcome, and (3) psychological consequences of SOs over time (i.e. patients’ uncertainty and anxiety). It was shown that cancer patients’ uncertainty was reduced directly after consulting for a SO, particularly if the first opinion was confirmed, whereas patients’ anxiety changed little over time. Thus, this study provides support for the potential psychological benefits of seeking a SO and challenges critics who suggested that SOs increase uncertainty [Citation2,Citation14,Citation15]. Nevertheless, the added medical value of SOs remains debatable, given that most patients and consulting oncologists perceived the SO as confirming the first opinion.
In line with previous research, patients’ most frequently reported motivations were related to hearing other experts’ opinions, receiving more information, and exhausting all options [Citation3,Citation4,Citation7,Citation8]. Although only a few participated, referring oncologists rather accurately recognized what motivated their patients to request a SO. However, as their inclusion was dependent on patients’ permission, referring oncologists with good insights into their patients’ motivations may have been over-selected. Nevertheless, 25% of patients sought the SO because they needed more information, whereas only 5% of oncologists anticipated this motivation. Thus, referring oncologists may not be fully aware of their patients’ information needs [Citation6,Citation24]. Oncologists may better explore how much and which information patients need when they request a SO and, in turn, seeking a SO may become unnecessary for some patients. Similarly, more and different referring oncologists than patients indicated they had advised their patients to seek an SO. This inconsistency in perceptions and/or communication between patients and oncologists warrants further investigation. Overall, larger-scale research focusing on referring oncologists is needed to further delineate our suggestions.
Patients’ expectations included hearing about different treatment options, having the first opinion confirmed, and/or receiving support in making treatment decisions, partially underlining their efforts to actively taking part in their own treatment plans. Yet, patients’ most frequently endorsed expectation (i.e. to hear something different) was rarely met, given that only 7 patients and 5 consulting oncologists reported a largely/completely different medical outcome of the SO. This suggests that patients may have unrealistic expectations, but that they could also be better informed about the nature and potential benefits of SOs. They should be made aware of the existence of (inter)national treatment protocols/guidelines, and about discussions of their specific diagnosis/treatment in multidisciplinary tumor boards [Citation6]. This can help patients to more realistically manage their expectations and to make better-informed decisions about whether or not to pursue a SO. More importantly, the identified lack of medical discrepancies between first and second opinions in the current study, suggests a limited medically added value of SOs. In contrast, recent retrospective studies in other oncology settings (e.g. breast cancer surgery) suggest much higher discrepancy rates between first and second opinion [Citation25,Citation26]. Given our setting in medical oncology, most patients had advanced cancer and were treated with palliative intent. This can imply limited treatment options and, thus fewer discrepancies in medical opinions relative to patients with early stage cancer. Alternatively, an additional explanation may be methodological differences: Even if patients and consulting oncologists perceived the first and second opinion as similar, a detailed retrospective analysis of medical records might still yield (minor) discrepancies. Additional research is needed that also includes more detailed clinical data and that establishes whether outcomes of objective retrospective medical record reviews are reflected in patients’ and oncologists’ perceptions. Such studies will help to identify implications of potential discrepancies for the clinical practice, and to test whether discrepancies between first and second opinions actually affect patients’ objective health and/or survival. Such future studies should take into account that even if most patients do not benefit medically, individual patients (who receive a largely different diagnosis/treatment due to a SO) may benefit immensely.
Irrespective of medical outcomes, the future debate about the added value of SOs should consider potential psychological benefits. This study identified stable levels of anxiety and considerably decreased uncertainty directly following the SO, indicating it can offer patients peace of mind. Even if the SO differed from the first opinion, patients’ uncertainty did not increase, suggesting that new/conflicting information does not necessarily induce confusion or uncertainty. Nevertheless, the reduction in uncertainty did not sustain over time (to T2), suggesting that uncertainty may be influenced by additional factors over time. Overall, our findings fuel the debate as to whether limited psychological benefits justify the resources, time, and financial burden of SOs. Alternative (referral) procedures and structures of SOs may be more cost-effective/efficient, while providing comparable psychological benefit to patients [Citation27]. Moreover, these alternatives may prove particularly useful when physical consultations are inconvenient or impossible, due to extensive travel time or restrictions like during the global Covid-19 pandemic. For example, in online SO platforms, patients can consult an independent specialist online [Citation25]. This potentially reduces time, effort, and costs for both patients and consulting oncologists. Patients have been found to primarily use this service to enhance their understanding of disease-related information provided by their own physician [Citation28]. Thus, for patients motivated by such an information need (∼25% in this study), a digital variant of a SO may be an adequate alternative. For patients motivated by a need for confirmation of the first opinion, an in-person SO may also be replaced by simpler variants. For example, referring oncologists could consult an independent specialist at an expert center (e.g. by phone) and relay the conclusions based on both opinions to their patient. Nevertheless, for some patients a face-to-face SO may remain their optimal way to acquire peace of mind and support their feeling of taking part in decision-making, feeling supported in choosing between options, and having exhausted all options. Upon SO request, referring oncologists could explicitly explore patients’ motivations and expectations to assess which SO variant would be most beneficial and to manage patients’ expectations.
Although providing novel knowledge in a prospective design, certain limitations of this study should be considered. Our sample size did not allow for extensive subgroup analyses although our efforts to maximize patient inclusion, achieving our a priori established sample size, and completion rates above 70% over three measurement points can be highlighted as positive. Notably, dropped-out patients did not differ regarding their sex, level of education, or age compared to retained participants. Yet, some effects may be more pronounced in larger samples, and insights into patients’ reasons for discontinuing participation are missing. One contributing factor may be our medical oncology setting, which led to including predominantly patients with advanced disease. Their ability/energy to participate may be limited, but they represent a particularly vulnerable group of cancer patients in need of optimal communication and care. The inclusion of referring oncologists may be highlighted as an asset of this study, but given the limited sample size and having their inclusion depend on patient approval warrants additional research. Moreover, highly educated patients were overrepresented in the current study relative to a generic oncology setting, but SOs are requested more often by higher educated people [Citation29–31]. This warrants additional research in diverse oncology settings, as well as different cultural contexts and countries with different healthcare systems.
To summarize, our results suggest that seeking a SO may yield psychological benefits for patients with advanced cancer, although medical benefits may be limited. For clinical practice, our results indicate that (referring) oncologists could inform patients better about potential goals of a SO to manage their expectations. Our results provide support for proponents’ views that SOs appear to do no harm and could benefit patients psychologically. At the same time, our findings support critics’ views that SOs may have little added medical value. In the medical community and beyond, an ongoing debate and more research are essential to further determine whether and how SOs can be optimized.
Disclosure statement
All authors, apart from F.Y.F. de Vos, declare no conflict of interest. Dr. de Vos specifies that he received personal grants from Roche and AbbVie, which are however independent of this work. All authors further state that views expressed in this manuscript are their own and not an official position of their institution or the funder.
Additional information
Funding
References
- Hillen MA, Medendorp NM, Daams JG, et al. Patient-driven second opinions in oncology: a systematic review. Oncologist. 2017;22(10):1197–1211.
- Ruetters D, Keinki C, Schroth S, et al. Is there evidence for a better health care for cancer patients after a second opinion? A systematic review. J Cancer Res Clin Oncol. 2016;142(7):1521–1528.
- Tattersall MHN, Dear RF, Jansen J, et al. Second opinions in oncology: the experiences of patients attending the Sydney Cancer Centre. Med J Aust. 2009;191(4):209–212.
- Philip J, Gold M, Schwarz M, et al. Second medical opinions: the views of oncology patients and their physicians. Support Care Cancer. 2010;18(9):1199–1205.
- Van De Plas J, Buntinx F, De Vadder I, et al. Cancer patients looking for a second opinion. [Dutch] [Kankerpatienten op zoek naar een tweede opinie: Frequentie en redenen van tweede opinies na een kankerdiagnose.]. Tijdschrift Voor Geneeskunde. 2010;66(16):770–774.
- Mellink WA, Dulmen AM, Wiggers T, et al. Cancer patients seeking a second surgical opinion: results of a study on motives, needs, and expectations [Research Support, Non-U.S]. J Clin Oncol. 2003;21(8):1492–1497.
- Radhakrishnan A, Grande D, Mitra N, et al. Second opinions from urologists for prostate cancer: who gets them, why, and their link to treatment. Cancer. 2017;123(6):1027–1034.
- Fuchs T, Hanaya H, Seilacher E, et al. Information deficits and second opinion seeking – a survey on cancer patients. Cancer Invest. 2016;28:1–8.
- King SB. Second opinion rights. JACC Cardiovasc Interv. 2014;7(9):1079–1080.
- Axon A, Hassan M, Niv Y, et al. Ethical and legal implications in seeking and providing a second medical opinion. Dig Dis. 2008;26(1):11–17.
- Hillen MA, Woei-A-Jin FSH, Smets EM, et al. Assessment of challenges encountered by dutch oncologists when patients ask for second opinions. JAMA Oncol. 2018;4(10):1425–1426.
- Moumjid N, Gafni A, Bremond A, et al. Seeking a second opinion: do patients need a second opinion when practice guidelines exist? Health Policy. 2007;80(1):43–50.
- Sikora K. Second opinions for patients with cancer. BMJ. 1995;311(7014):1179–1180.
- Maaskant JM, Van Muilekom H. The first doctor is seldom wrong [Dutch]. Medisch Contact. 2009;64(14):605–607.
- Mellink WA, Henzen-Logmans SC, Bongaerts AH, et al. Discrepancy between second and first opinion in surgical oncological patients [Comparative Study]. Eur J Surg Oncol. 2006;32(1):108–112.
- Hillen MA, Gutheil CM, Smets EMA, et al. The evolution of uncertainty in second opinions about prostate cancer treatment. Health Expect. 2017;20(6):1264–1274.
- Von Elm E, Altman DG, Egger M, STROBE Initiative, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577.
- Mishel MH. The measurement of uncertainty in illness. Nurs Res. 1981;30(5):258–263.
- Mishel MH. Adjusting the fit: development of uncertainty scales for specific clinical populations. West J Nurs Res. 1983;5(4):355–370.
- Blanch-Hartigan D, van Eeden M, Verdam MG, et al. Effects of communication about uncertainty and oncologist gender on the physician-patient relationship. Pat Educ Couns. 2019;102(9):1613–1620.
- Spielberger CD. Assessment of state and trait anxiety: conceptual and methodological issues. South Psychol. 1985;2:6–16.
- van der Bij AK, de Weerd S, Cikot R, et al. Validation of the Dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genet. 2003;6(2):84–87. 2003
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- Goldman RE, Sullivan A, Back AL, et al. Patients' reflections on communication in the second-opinion hematology-oncology consultation. Patient Educ Couns. 2009;76(1):44–50.
- Schook RMtA MJ, van Setten CH, de Man FF, et al. Lung cancer patients benefit from second opinions by improvement of diagnosis and therapy. Cancer Clin Oncol. 2014;3(1):43–57.
- Heeg E, Civil Y, Hillen M, et al. Impact of second opinions in breast cancer diagnostics and treatment: a retrospective analysis. Ann Surg Oncol. 2019;26(13):4355–4359.
- Hillen MA, Smets EMA, Woei-A-Jin F, et al. Second opinions op verzoek van de patiënt – de visie van medisch oncologen en hematologen [Patient-initiated second opinions – opinions of medical oncologists and hematologists]. Ned Tijdschr Oncol [Dutch Journal for Oncology]. 2019;16:131–138.
- Schook RM, Linssen C, Schramel FM, et al. Why do patients and caregivers seek answers from the Internet and online lung specialists? A qualitative study. J Med Internet Res. 2014;16(2):e37.
- Tam KF, Cheng DK, Ng TY, et al. The behaviors of seeking a second opinion from other health-care professionals and the utilization of complementary and alternative medicine in gynecologic cancer patients. Support Care Cancer. 2005;13(9):679–684.
- Mordechai O, Tamir S, Weyl-Ben-Arush M. Seeking a second opinion in pediatric oncology. Pediatr Hematol Oncol. 2015;32(4):284–289.
- Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556.
