Abstract
Background
In colorectal cancer (CRC) patients, guidelines only recommend measurement of preoperative carcinoembryonic antigen (CEA), although postoperative CEA may be more informative. However, the sensitivity of both preoperative and postoperative CEA in identifying relapse is limited. We studied whether CA19-9, YKL-40, C-reactive protein (CRP) and interleukin (IL)-6 add prognostic information combined with postoperative CEA.
Material and methods
This post-hoc analysis included 147 radically resected stage II (n = 38), III (n = 91) and IV (n = 18) CRC patients treated with adjuvant 5-fluorouracil (5-FU)-based therapy in the phase III LIPSYT study (ISRCTN98405441). We collected postoperative blood samples a median of 48 days after surgery. We analysed relapses, sensitivity, positive predictive value (PPV) and disease-free (DFS) and overall survival (OS) by bootstrap, Kaplan–Meier and adjusted Cox-models in the elevated vs. normal biomarker groups.
Results
Elevated postoperative CEA associated with impaired DFS (HR 7.23; CI95% 3.85–13.58), impaired OS (HR 7.16; CI95% 3.76–13.63), and more relapses (HR 7.9; CI95% 3.4–18.2); but sensitivity for CEA in finding relapses was only 31% (CI95% 21–48%). Normal CEA combined with an elevated YKL-40 or elevated CRP showed more relapses (HR for YKL-40 2.13 [CI95% 1.10–4.13], HR for CRP 3.14 [CI95% 1.21–8.16]), impaired DFS (HR 2.18 [CI95% 1.12–4.24] or 3.23 [CI95% 1.34–7.82]), and impaired OS (2.33 [CI95%1.24–4.40] or 2.68 [CI95%1.12–6.44]). Elevated CEA combined with a concomitantly elevated CA19-9, YKL-40, CRP or IL-6 showed a respective PPV of 100, 90, 100, and 100%.
Conclusion
In radically operated stage II to IV CRC patients who received adjuvant 5-FU-based chemotherapy, a postoperatively elevated CEA alone or in combination with CA19-9, YKL-40, CRP, or IL-6, or a normal CEA combined with an elevated YKL-40 or with an elevated CRP, may indicate patients at high risk of relapse.
Introduction
Treatment of choice for locoregional colorectal cancer (CRC) and resectable metastases is surgery with curative intent. Despite improvements achieved in surgery and in adjuvant chemotherapy, 30–50% of stage II–III patients and 50% with resected stage IV disease will develop a recurrence [Citation1–4].
There is a need for biomarkers to determine patients’ risk for recurrence; routine high-risk factors have proven unsatisfactory [Citation1,Citation3,Citation4]. Several new multigene assays provide individualised prognostic information, and liquid biopsies are emerging as a method to detect minimal residual disease. Yet, methodologically, these are challenging technologies, and data to support their use in everyday clinical practice are insufficient [Citation3–5].
Blood-based biomarkers are easy to sample and could be ideal for evaluating the risk of cancer recurrence. CEA, carcinoembryonic antigen, is a tumour-associated glycoprotein, rather than a tumour-specific marker. For years, CEA has been the only widely recommended biomarker for CRC prognostics and follow-up [Citation6,Citation7]. However, the sensitivity of preoperative CEA in detecting CRC recurrence is only 50–80%, and the specificity 80% [Citation8]. There are studies showing that postoperative CEA is more informative than preoperative CEA [Citation9,Citation10]. New biomarkers are therefore essential to improve CEA’s clinical utility.
CA19-9, carbohydrate antigen 19-9, is a widely used marker in gastrointestinal cancers [Citation11], but its prognostic value after resection of CRC is less established [Citation6,Citation7]. CRC is associated with inflammation, which promotes carcinogenesis and tumour growth [Citation12]. Therefore, inflammation-related biomarkers or mediators such as YKL-40 (also known as human cartilage glycoprotein-39 or chitinase-3-like-1 protein), C-reactive protein (CRP), and interleukin (IL)-6 have emerged as prognostic biomarkers. YKL-40 is a secreted protein produced by non-malignant cells such as macrophages and by cancer cells such as colon cancer cells [Citation13]. YKL-40 promotes cancer proliferation and inflammatory cytokine production [Citation14]. CRP is a non-specific acute-phase reactant reflecting tissue damage and is also a sensitive and stable marker of inflammation [Citation15]. In cancer patients, including CRC, elevated serum CRP is associated with inflammation, cachexia, and poor prognosis both in metastatic and localised disease [Citation16]. IL-6, mainly produced by monocytes and macrophages, among others, as well as by various tumour cells [Citation17] stimulates YKL-40 production and angiogenesis; it is regarded as an important tumour-promoting factor in various human cancers, including CRC [Citation18]. Our aim was to evaluate the prognostic value of postoperative CA19-9, YKL-40, CRP, and IL-6 in combination with CEA in radically resected stage II–IV CRC patients receiving adjuvant chemotherapy.
Material and methods
The original LIPSYT trial was an open-label, prospective, randomised single-institution phase III study in patients with radically resected CRC (http://www.controlled-trials.com/ISRCTN98405441). Patients included received treatment at the Department of Oncology of Helsinki University Hospital (Finland) between November 1997 and August 2001. That study’s primary aim was to assess treatment tolerability in a two-by-two factorial design; the secondary aim was to study biomarkers.
This is a post-hoc analysis of the LIPSYT trial, with 150 patients, of which 3 never started treatment. Inclusion criteria were age 18 to 75 years, histologically confirmed radically resected stage II–IV CRC (radical metastasectomy of mostly liver metastases, n = 18), WHO performance status 0–2 and adequate bone-marrow-, kidney-, and liver-function. Exclusion criteria included a history of invasive cancer other than CRC; metabolic, neurological, or psychiatric illness incompatible with chemotherapy; serious thromboembolic event currently under treatment; pregnancy, lactation, or absence of adequate contraception in fertile patients. The protocol was approved by an institutional review board and all study participants gave their signed informed consent. Adjuvant chemotherapy consisted of 5-FU and LV as a bolus injection (Mayo regimen) or continuous infusion (simplified de Gramont regimen) according to randomisation [Citation19].
Assessment of biomarkers
CEA, YKL-40, CRP, IL-6 and CA19-9 were measured in serum samples collected postoperatively before adjuvant chemotherapy began. Median time from surgery to laboratory sampling and treatment initiation was 48 days (range 19–124) and more than 8 weeks in 40 (27%). The reason for treatment delay was referral-delay to oncology for 33 patients, and 7 patients being unfit to start oncologic treatments within 8 weeks.
CRP, CEA, and CA19-9 were determined by the routine laboratory of the university hospital with automatic analysers as follows: CRP: immunoturbidimetric method, CEA and CA19-9: immunoenzymatic assay, Bayer Immuno 1 (CEA: 10/1998–10/2005; and CA19-9: 1/1998–1/2006), or immunochemiluminometric assay, Abbott Architect (CEA: 10/2005→ and CA19-9: 1/2006→). All measurements were performed by technicians blinded to study endpoints.
Blood samples for YKL-40 and IL-6 were collected in gel tubes and centrifuged within 2 h; serum was stored at −20 °C until analysis. YKL-40 and IL-6 were determined in duplicate with commercially available enzyme-linked immunosorbent assays (ELISAs); YKL-40: MicroVue YKL-40 ELISA (Catalog #8020), Quidel Corporation, San Diego, CA, USA; and IL-6: Quantikine HS600B, R&D Systems, Abingdon, OX, UK; according to manufacturer’s instructions. For YKL-40, the detection limit was 20 ng/ml and intra- and inter-assay coefficients of variation (CVs) of <5% and <6% [Citation17]. For IL-6, the detection limit was 0.01 pg/ml and intra- and inter-assay CVs of ≤8% and ≤11% [Citation20].
An age-corrected percentile for YKL-40 was calculated according to the formula [Citation17]: percentile = 100/(1 + (YKL-40−3) × (1.062age) × 5000). Cut-off values were: ROC corrected cut-off for age-corrected YKL-40 level as the 70.7 percentile of normal controls YKL-40 (see statistics), 4.5 pg/ml for IL-6 [Citation20]; 10 mg/l for CRP; 5 µg/l for CEA; and 26 kU/l for CA19-9 according to the clinical routine at HUSLab, Helsinki, Finland.
Statistics
Clinicopathological parameters and tumour-marker values are presented as frequencies or medians with range for nonparametric distributions. The chi-squared test served for comparisons between categorical variables, and the Mann–Whitney or Kruskall–Wallis for non-normally distributed continuous variables.
Time-dependent receiver-operating characteristic (ROC) curves were constructed for DFS at 5 years of follow-up, and area under the curve (AUC) values determined (timeROC package in R, https://cran.r-project.org/web/packages/timeROC/v0.3 by Paul Blanche). The cut-off value for YKL-40 (70.7) was obtained by maximising Youden’s index at 5 years of follow-up, giving sensitivity, and specificity equal weight.
Descriptive survival analysis was performed with the Kaplan–Meier estimator, and the log-rank test served to compare groups. Disease-free survival (DFS) was defined as a composite endpoint of recurrence or death from any cause with censoring at the last date of follow-up at 10+ years from postoperative sampling. The endpoint for overall survival (OS) was death from any cause with censoring at the last date of follow-up. Median follow-up time for patients alive was 11.9 (range 8.9–12.7) years. Unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CI95%) were estimated with the Cox regression proportional hazard model. Firth’s penalised maximum likelihood bias correction method for Cox regression was used if mentioned.
The adjustment was for age, sex, inflammatory disease and stage in univariate analysis and further for CEA, CA19-9, YKL-40, CRP and IL-6 in multivariate models. The inflammatory disease was a history of autoimmune diseases such as rheumatoid arthritis, iritis, psoriasis, ulcerative colitis, coeliac disease and thyroiditis in 14 patients. None had active ulcerative colitis. For the multivariate model, all biomarkers with background variables were included (into the model). Relapse events at 7 years were defined as CRC recurrence (distant or local), new CRC, or death from CRC, but new non-CRC cancer or deaths from other causes were not events. Specificity, sensitivity, and positive predictive value (PPV) for a biomarker being elevated vs. normal were calculated for relapse at 7 years by using non-colorectal deaths as competing events. The 95% bias-corrected accelerated CIs (BCa) were obtained by a bootstrapping method with 1000 replications (boot package in R [Citation21]). The Clopper–Pearson confidence interval was calculated for PPV in subgroup analysis. Relapse was analysed with the Cox proportional hazards regression, with other deaths as competing risks, and adjusted for age (median cut off), sex, inflammatory disease, and TNM stage. Subgroup analyses are, however, unadjusted.
The statistical significance level was set at 5%; all tests were two-sided. Statistical analyses were done with SPSS version 24.0 (IBM SPSS Statistics, version 22.0 for Mac; SPSS, Inc., Chicago, IL, USA), R version 3.6.1 (Foundation for Statistical Computing, Vienna, Austria), SAS for Windows (v9.4, SAS Institute Inc., Cary, NC, USA) and STATA/MP (v.15.1, StataCorp LLC, College Station, TX, USA).
Results
Baseline characteristics and biomarker levels
The 147 patients were of median 60 years (range 31–76 years) and most presented with a locoregional disease (88% stage II–III, ). After 10 years, 77 (52%) were still alive. Relapse was detectable in 65 (44%), with no new relapses after 6.3 years. Cause of death was metastatic CRC (mCRC) in 84% (58/70), cardiovascular in 10% (7/70), second cancer in 3% (2/70), and other cause in 3% (2/70). Levels of CEA, CA19-9, YKL-40 or CRP, according to the primary location (right colon vs. left colon vs. rectum), showed no differences, but IL-6 was higher in patients with rectal cancer ().
Table 1. Patient characteristics and postoperative biomarker levels.
CEA
Elevated CEA showed an increased hazard of relapse; the HR adjusted for background variables was 7.91 (CI95% 3.43–18.24, other deaths as competing risk; ). For CEA elevated vs. normal, the sensitivity was 31% (CI95% 21–48%), specificity was 97% (CI95% 91–100%), and PPV was 89% (CI95% 65–99%; ).
Table 2. Number of patients with no relapse vs. relapse with or without CRC death and other competing non-CRC deaths, assessed at 7 years.
In univariate analysis adjusted for baseline characteristics, elevated CEA associated with impaired DFS (HR 7.23, CI95% 3.85–13.58; 10-year DFS rate 6% for elevated CEA vs. 63% for normal CEA; , ) and impaired OS (HR 7.16, CI95% 3.76–13.63; 10-year OS rate 6% for elevated CEA vs. 68% for normal CEA; , ).
Figure 1. Disease-free survival (DFS) (A) and overall survival (OS) (B) in postoperatively elevated vs. normal CEA.
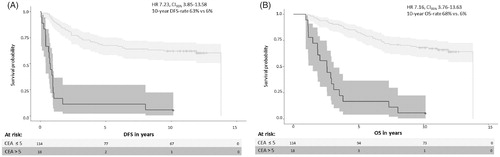
Table 3. Univariate and multivariate analysis for DFS and OS.
In multivariate analysis, elevated CEA associated with impaired DFS (HR 8.63, CI95% 3.82–19.50) and impaired OS (HR 10.17, CI95% 4.35–23.79; ).
CA19-9
Elevated CA19-9 levels vs. normal had an adjusted HR of 2.08 (95% CI 0.87–4.98) for relapse, using other deaths as competing events. Elevated vs. normal CA19-9 showed sensitivity of 16% (CI95% 8–27%), specificity 89% (CI95% 79–95%), and PPV 53% (CI95% 27–78%, ). No association appeared between elevated CA19-9 and DFS or OS (), which was true also for the subgroup with elevated CA19-9 and normal CEA.
All five patients with concomitantly elevated CEA and CA19-9 relapsed (PPV 100%, CI95% 48–100%, the Clopper–Pearson interval), had a numerically shorter DFS (0.41 vs. 0.76 years, HR 3.16 CI95% 0.82–14.0, with Firth’s bias correction) and significantly shorter OS (1.8 vs. 2.9 years, HR 4.46; CI95% 1.1–20.0, Firth’s bias correction), compared to patients with either elevated or both normal.
YKL-40
Elevated YKL-40 showed an increased hazard of relapse; HR adjusted for background variables being 1.73 (CI95% 1.02–2.93, other deaths as competing risk). The sensitivity for finding relapses was 56% (CI95% 43–69%), specificity 69% (CI95% 58–79%), and PPV 57% (CI95% 45–69%; ). In univariate analysis, elevated YKL-40 vs. normal associated with impaired DFS (HR 1.84, CI95% 1.14–2.96, 10-year DFS rate 37 vs. 59%) and impaired OS (HR 1.97, CI95% 1.20–3.23, 10-year OS rate 43 vs. 64%; ). In adjusted multivariate analysis, elevated YKL-40 associated with impaired OS (HR 2.24, CI95% 1.23–4.05), but not with impaired DFS (HR 1.58, CI95% 0.88–2.86; ).
In the subgroup with normal CEA, elevated YKL-40 had an unadjusted HR of 2.13 (95% CI 1.10–4.13) for relapse, impaired DFS (HR 2.30, CI95% 1.27–4.16; ), and OS (HR 2.40, CI95% 1.28–4.52; ) compared with normal YKL-40. Patients with normal CEA and elevated YKL-40 had a 10-year DFS rate of 49 vs. 71% in patients with both normal, and a 10-year OS rate of 55 vs. 77%.
Figure 2. The subgroup with normal postoperative CEA and elevated YKL-40 with disease free survival (DFS) (A), overall survival (OS) (B), and the subgroup with normal postoperative CEA and elevated CRP with DFS (C) and OS (D).
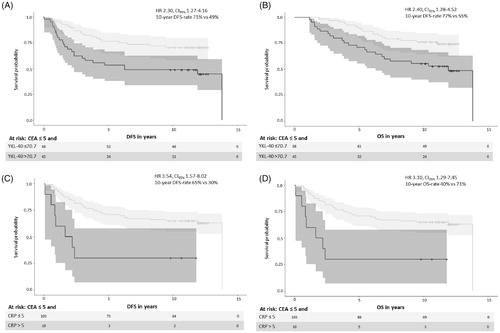
In the small subgroup with concomitant elevation of both YKL-40 and CEA, 9 of 10 patients without competing deaths had a relapse (PPV 90%, CI95% 56–100%, Clopper–Pearson interval; ), numerically shorter DFS (0.68 vs. 0.76 years, HR 0.82, CI95% 0.31–2.15) and numerically shorter OS (1.5 vs. 2.6 years, HR 1.32, CI95% 0.51–3.44) than did patients with either elevated or both normal.
CRP
Elevated CRP levels vs. normal levels had an adjusted HR of 1.95 (CI95% 0.97–3.94) for relapse using other deaths as competing events. Elevated CRP showed sensitivity of 20% for finding relapses (CI95% 12–30%), specificity of 96% (CI95% 89–99%) and PPV of 77% (CI95% 44–93%; ). In univariate analysis, elevated vs. normal CRP associated with impaired DFS (HR 2.31, CI95% 1.21–4.39; 10-year DFS rate 18 vs. 53%) and impaired OS (HR 2.40, CI95% 1.20–4.80; 10-year OS rate 24 vs. 59%). In adjusted multivariate analysis, elevated CRP associated with impaired DFS (HR 2.53; CI95% 1.10–5.81), but not with OS (HR 2.49; CI95% 0.95–6.51; ).
In the subgroup with normal CEA and elevated CRP, unadjusted HR for relapse was 3.14 (CI95% 1.21–8.16). Patients with normal CEA and elevated CRP had an impaired DFS (HR 3.54, CI95% 1.57–8.02; ) and an impaired OS (HR 3.10, CI95% 1.29–7.45; ) compared with patients with normal CEA and normal CRP. Patients with normal CEA and elevated CRP had a 10-year DFS rate of 30 vs. 65% with normal CEA and normal CRP, and a 10-year OS rate of 40 vs. 71%.
All four patients with concomitant elevation of CEA and CRP had a relapse (PPV 100%, CI95% 40–100%; ), numerically shorter DFS (0.39 vs. 0.76 years, HR 3.13, CI95% 0.90–10.90), and numerically shorter OS (1.8 vs. 2.6 years, HR 1.52, CI95% 0.48–4.77) than did patients with either elevated or both normal.
IL-6
Elevated IL-6 predicted increased hazard of relapse; HR adjusted for background variables was 2.12 (CI95% 1.11–4.03, other deaths as competing risk). Sensitivity for elevated IL-6 in finding relapses was 28% (CI95% 17–40%), specificity 91% (CI95% 84–97%) and PPV 75% (CI95% 53–90%; ). In univariate analysis, elevated IL-6 associated with impaired OS (HR 1.99, CI95% 1.05–3.76; 10-year OS rate for elevated IL-6 vs. normal IL-6 was 33 vs. 58%) but not with impaired DFS (HR 1.77, CI95% 0.95–3.30, 10-year DFS rate for elevated IL-6 vs. normal IL-6 being 25 vs. 53%; ). In multivariate analysis, IL-6 was not an independent biomarker of DFS or OS ().
Normal CEA and elevated IL-6 did not associate with impaired DFS (HR 1.36, CI95% 0.53–3.51) or impaired OS (HR 1.30, CI95% 0.48–3.51).
All six patients with concomitantly elevated CEA and IL-6 relapsed (PPV 100%, CI95% 54–100%) and had a numerically shorter DFS (0.41 vs. 0.76, HR 2.16, CI95% 0.75–6.16) and OS (1.1 vs. 2.6, HR 1.96, CI95% 0.71–5.44) than did patients with either elevated or both normal.
Discussion
The present study demonstrates that postoperatively elevated serum CEA, YKL-40, CRP, and IL-6 predict recurrence and impaired survival, but in adjusted multivariate analysis only CEA remained significantly associated with both impaired DFS and impaired OS. In patients with normal CEA and elevated YKL-40 or CRP, their DFS and OS were impaired. If elevated CEA was combined with elevated CA19-9, YKL-40, CRP, or IL-6, the risk of CRC relapse was very high (90–100%) and the OS short. If validated, these elevated biomarkers, combined with CEA, may prove useful for identifying patients suitable for a more intensive follow-up to earlier identify resectable metastases, and in identifying low-risk patients who may need adjuvant chemotherapy or high-risk who need the addition of oxaliplatin.
According to guidelines, CEA is the only biomarker that should be routinely measured in patients with CRC [Citation6,Citation7]. Elevated preoperative CEA has been shown to be an important prognostic factor in addition to the TNM stage and other known prognostic factors [Citation22,Citation23]. However, both the sensitivity and specificity of CEA are limited, and a notable part of high-risk patients can remain undetected if only CEA is measured [Citation8,Citation24,Citation25]. In the Cochrane review, CEA was insufficiently sensitive, even with low thresholds (<2.5) and they concluded that a rise in CEA never occurs in up to 20% of patients with a true recurrence (false negatives) and thus multiple modalities are recommended [Citation26]. Postoperative CEA is better than preoperative in some studies [Citation9,Citation10]. There exists a clear need for a more precise combination of biomarkers.
CA19-9 is a biomarker widely used in gastrointestinal cancers including CRC; an elevated level has been a negative prognostic marker in mCRC [Citation27] and in localised CRC [Citation28–30]. Our patients with both postoperatively elevated CEA and CA19-9 relapsed and had short DFS and OS, but CA19-9 alone was not an independent predictor of relapse and survival in CRC. Some findings are contrary to ours [Citation30,Citation31], but findings in line with ours imply that CA19-9 has potential value in combination with elevated CEA [Citation29,Citation32,Citation33]. More convincing data are essential before the adoption of CA19-9 surveillance into clinical practice.
Chronic inflammation plays an important role in colorectal carcinogenesis and is associated with poor prognosis [Citation12], but data concerning YKL-40 as a prognostic postoperative biomarker in CRC after radical surgery are limited [Citation34]. YKL-40 has been elevated in CRC patients and more sensitive than CEA and CA19-9, especially in localised CRC [Citation35–37]. High levels of pre-treatment YKL-40 are associated with short OS in chemotherapy for mCRC [Citation38], and in patients undergoing liver resection [Citation39]. We demonstrate that in radically resected CRC patients, high YKL-40 is a predictor of impaired OS. Furthermore, patients with normal CEA but with elevated YKL-40 had impaired DFS (10-year DFS rate estimate 49 vs. 71%) and OS (10-year OS rate 55 vs. 77%). Our findings are in line with those of Cintin et al. [Citation34] showing postoperatively elevated YKL-40 to be an independent predictor of higher recurrence rates and impaired survival in stage II–IV disease. Our patients with concomitantly elevated CEA and YKL-40 had a 90% recurrence rate. As YKL-40 predicts survival regardless of the CEA level, it may add additional information to CEA measurement and improve risk stratification.
Discordant prognostic significance, especially for preoperative CRP, is evident in one comprehensive review [Citation40]. We showed that elevated postoperative CRP associated with impaired DFS and OS in univariate analysis, in line with earlier findings [Citation41,Citation42]. Our patients with normal CEA and elevated CRP had significantly worse 10-year DFS rates (30 vs. 65%) and OS rates (40 vs. 71%) than patients with both markers normal. In fact, all with postoperatively elevated CEA and CRP relapsed. Accordingly, CRP is a strong predictor of poor survival, a complementary one to CEA.
Data concerning IL-6 as a prognostic biomarker in CRC are ambiguous according to one review, and in most studies presented IL-6 was not an independent prognostic biomarker [Citation43]. Our results are in accordance with these findings as IL-6 was prognostic only in univariate analysis for OS, but not in multivariate analysis. In a study with stage I-III CRC patients, those with high preoperative IL-6, had significantly shorter DFS [Citation44], which is in line with our univariate analysis. All our patients with concomitantly elevated CEA and IL-6 recurred.
Postoperatively elevated CEA is prognostic for relapse [Citation9,Citation10], in line with our findings, and identifies patients in need for more intensive adjuvant therapy [Citation45,Citation46]. Stage II colon cancer patients with elevated postoperative CEA benefit from adjuvant therapy vs. surgery alone, or from more intensified therapy with the addition of oxaliplatin [Citation46,Citation47].
Among the strengths of our study are that all patients underwent high-quality surgery followed by long-term monitoring. All received adjuvant therapy with 6 months of 5-FU, although without oxaliplatin. A further strength is the follow-up routine with clinical examination, routine laboratory measurements, including tumour markers, and radiology for 10 years within the prospective study at a single institution. Limitations are the small sample sizes in subgroup analyses, resulting in limited statistical power. Secondly, as routine CEA, CA19-9, and CRP were not systematically analysed preoperatively, we, therefore, could not study persistently elevated markers postoperatively vs. markers that postoperatively normalised. Another limitation is that some sampling of biomarkers and initiation of adjuvant therapy was delayed beyond 8 weeks, perhaps impacting results of the efficacy of adjuvant therapy but will probably not have had an influence on the prognostic effect of the postoperative markers. These patients were treated in 1997–2001 with 5-FU-based chemotherapy and the results should be validated in patients treated with oxaliplatin-based chemotherapy.
In conclusion, we showed that post-operatively elevated CEA alone or in combination with elevated YKL-40, CRP, CA19-9, or IL-6, associate with high recurrence rates in radically resected stage II-IV CRC patients. In addition, postoperative normal CEA in combination with elevated YKL-40 or CRP may indicate a patient’s high risk of relapse.
Acknowledgments
The authors thank Ulla Kjærulff-Hansen and Marianne Sørensen (Department of Medicine, Herlev and Gentofte Hospital, Copenhagen University Hospital, Denmark) and Mie Barthold Krüger (Department of Oncology, Herlev and Gentofte Hospital, Copenhagen University Hospital, Denmark) for technical assistance with the YKL-40 and IL-6 ELISA measurements. The authors give thanks to Elina Aspiala for technical assistance and to Carol Norris for proofreading.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):vi64–vi72.
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683.
- Benson AB, Venook A, Al-Hawary MM, et al. NCCN guidelines for rectal cancer. NCCN Guidelines. 2020 Version 4. 2020 [cited 2020 Jun 3].
- Benson AB, Venook A, Al-Hawary MM, et al. NCCN guidelines for colon cancer. NCCN Guidelines. 2020 Version 3. 2020 [cited 2020 Jun 3].
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
- Duffy MJ, van Dalen A, Haglund C, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43(9):1348–1360.
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313–5327.
- Sorensen CG, Karlsson WK, Pommergaard HC, et al. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence – a systematic review. Int J Surg. 2016;25:134–144.
- Lin JK, Lin CC, Yang SH, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26(9):1135–1141.
- Konishi T, Shimada Y, Hsu M, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309–315.
- Scara S, Bottoni P, Scatena R. CA 19-9: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:247–260.
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and Colon Cancer. Gastroenterology. 2010;138(6):2101.e5–2114.e5.
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172–209.
- Yeo IJ, Lee CK, Han SB, et al. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019;203:107394.
- Shrotriya S, Walsh D, Nowacki AS, et al. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS One. 2018;13(8):e0202555.
- Kersten C, Louhimo J, Ålgars A, et al. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013;52(8):1691–1698.
- Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta. 2011;412(9–10):709–712.
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6-a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8(9):1248–1253.
- Osterlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97(8):1028–1034.
- Knudsen LS, Christensen IJ, Lottenburger T, et al. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers. 2008;13(1):59–78.
- Davison AC, Hinkley DV. Bootstrap methods and their applications. Cambridge (UK): Cambridge University Press; 1997.
- Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88(7):1739–1757.
- Thirunavukarasu P, Sukumar S, Sathaiah M, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103(8):689–697.
- Shinkins B, Nicholson BD, James T, et al. What carcinoembryonic antigen level should trigger further investigation during colorectal cancer follow-up? A systematic review and secondary analysis of a randomised controlled trial. Health Technol Assess. 2017;21(22):1–60.
- Yavorkovsky LL, Kwong MS, Jhatakia S, et al. Unrecognized value of carcinoembryonic antigen in recurrent rectal and sigmoid colon cancer: case series. Perm J. 2019;23. DOI:10.7812/TPP/18-022
- Nicholson BD, Shinkins B, Pathiraja I, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;12:Cd011134.
- Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, et al. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arh. 2013;67(6):397–401.
- Shin JK, Kim HC, Lee WY, et al. High preoperative serum CA 19-9 levels can predict poor oncologic outcomes in colorectal cancer patients on propensity score analysis. Ann Surg Treat Res. 2019;96(3):107–115.
- Shibutani M, Maeda K, Nagahara H, et al. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res. 2014;34(7):3753–3758.
- Zhou W, Yang F, Peng J, et al. High pretreatment serum CA19-9 level predicts a poor prognosis for patients with stage III colon cancer after curative resection and adjuvant chemotherapy. J Cancer. 2019;10(16):3810–3818.
- Ryuk JP, Choi GS, Park JS, et al. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014;86(3):143–151.
- Park IJ, Choi GS, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29(10):4303–4308.
- Abe S, Kawai K, Ishihara S, et al. Prognostic impact of carcinoembryonic antigen and carbohydrate antigen 19-9 in stage IV colorectal cancer patients after R0 resection. J Surg Res. 2016;205(2):384–392.
- Cintin C, Johansen JS, Christensen IJ, et al. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95(2):267–274.
- Ye HM, Lu YZ, Liang XM, et al. Clinical significance of combined testing of YKL-40 with CEA in Chinese colorectal cancer patients. Clin Lab. 2014;60(3):397–405.
- Johansen JS, Christensen IJ, Jorgensen LN, et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4,496 subjects at risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(3):621–626.
- Fuksiewicz M, Kotowicz B, Rutkowski A, et al. The assessment of clinical usage and prognostic value of YKL-40 serum levels in patients with rectal cancer without distant metastasis. Technol Cancer Res Treat. 2018;17. DOI:10.1177/1533033818765209
- Tarpgaard LS, Guren TK, Glimelius B, et al. Plasma YKL-40 in patients with metastatic colorectal cancer treated with first line oxaliplatin-based regimen with or without cetuximab: results from the NORDIC VII Study. PLoS One. 2014;9(2):e87746.
- Peltonen R, Gramkow MH. Elevated serum YKL-40, IL-6, CRP, CEA, and CA19-9 combined as a prognostic biomarker panel after resection of colorectal liver metastases. PLOS One. 2020.
- Guo YZ, Pan L, Du CJ, et al. Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev. 2013;14(1):243–248.
- Matsubara D, Arita T, Nakanishi M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020;25(4):602–613.
- Yamamoto M, Saito H, Uejima C, et al. Prognostic value of the combination of pre- and postoperative C-reactive protein in colorectal cancer patients. Surg Today. 2018;48(11):986–993.
- Vainer N, Dehlendorff C, Johansen JS. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget. 2018;9(51):29820–29841.
- Shiga K, Hara M, Nagasaki T, et al. Preoperative serum interleukin-6Is a potential prognostic factor for colorectal cancer, including stage II patients. Gastroenterol Res Pract. 2016;2016:9701574.
- Aubin JM, Bressan AK, Grondin SC, et al. Assessing resectability of colorectal liver metastases: how do different subspecialties interpret the same data? Can J Surg. 2018;61(4):251–256.
- Spindler BA, Bergquist JR, Thiels CA, et al. Incorporation of CEA improves risk stratification in stage II colon cancer. J Gastrointest Surg. 2017;21(5):770–777.
- Auclin E, Andre T, Taieb J, et al. Association of post-operative CEA with survival and oxaliplatin benefit in patients with stage II colon cancer: a post hoc analysis of the MOSAIC trial. Br J Cancer. 2019;121(4):312–317.
