Abstract
Background
Checkpoint inhibitors have changed overall survival for patients with advanced melanoma. However, there is a lack of data on health-related quality of life (HRQoL) of long-term advanced melanoma survivors, years after treatment. Therefore, we evaluated HRQoL in long-term advanced melanoma survivors and compared the study outcomes with matched controls without cancer.
Material and methods
Ipilimumab-treated advanced melanoma survivors without evidence of disease and without subsequent systemic therapy for a minimum of two years following last administration of ipilimumab were eligible for this study. The European Organization for Research and Treatment of Cancer quality of life questionnaire Core 30 (EORTC QLQ-C30), the Multidimensional Fatigue Inventory (MFI), the Hospital Anxiety and Depression Scale (HADS), and the Functional Assessment of Cancer Therapy-Melanoma questionnaire (FACT-M) were administered. Controls were individually matched for age, gender, and educational status. Outcomes of survivors and controls were compared using generalized estimating equations, and differences were interpreted as clinically relevant according to published guidelines.
Results
A total of 89 survivors and 265 controls were analyzed in this study. After a median follow-up of 39 (range, 17–121) months, survivors scored significantly lower on physical (83.7 vs. 89.8, difference (diff) = −5.80, p=.005), role (83.5 vs. 90, diff = −5.97, p=.02), cognitive (83.7 vs. 91.9, diff = −8.05, p=.001), and social functioning (86.5 vs. 95.1, diff = −8.49, p= <.001) and had a higher symptom burden of fatigue (23.0 vs. 15.5, diff = 7.48, p=.004), dyspnea (13.3 vs. 6.7, diff = 6.47 p=.02), diarrhea (7.9 vs. 4.0, diff = 3.78, p=.04), and financial impact (10.5 vs. 2.5, diff = 8.07, p=.001) than matched controls. Group differences were indicated as clinically relevant.
Discussion
Compared to matched controls, long-term advanced melanoma survivors had overall worse functioning scores, more physical symptoms, and financial difficulties. These data may contribute to the development of appropriate survivorship care.
Introduction
Advanced melanoma is an aggressive malignant disease with high mortality as a hallmark. The introduction of small molecule targeted therapies and checkpoint inhibitors have improved clinical outcomes substantially [Citation1,Citation2]. In particular, checkpoint inhibitors blocking CTLA-4 (e.g., ipilimumab) and PD-1 (e.g., nivolumab and pembrolizumab) have been shown to induce long-term survival with ongoing responses after treatment discontinuation [Citation3,Citation4]. As a consequence of this treatment success, a new group of cancer survivors has arisen. Moreover, immune checkpoint inhibitors (ICI) are currently moving to the (neo) adjuvant setting [Citation5–7], which is expected to expand the melanoma survivor population even further.
Despite these favorable outcomes, patients diagnosed with unresectable advanced melanoma still face a life-threatening diagnosis, and are confronted with unpredictable sequelae of ICI treatment.
In 2011, ipilimumab was the first ICI approved by the U.S. Food and Drug Administration for the treatment of advanced melanoma. Several studies have evaluated the efficacy and on-treatment tolerability of ipilimumab [Citation8–10]. Although ipilimumab is frequently associated with adverse events, such as dermatological and gastrointestinal toxicities, most of these, except those due to endocrinological immune-related toxicities, are largely reversible [Citation11]. The impact of ipilimumab on HRQoL during the induction phase has only been evaluated in few studies and the results of these studies were not unequivocal [Citation12-19].
Insight into physical, psychological, and social morbidity of this new and growing group of cancer survivors is of paramount importance as it could contribute to the development of appropriate and adequate survivorship care for advanced melanoma survivors. Therefore, we initiated a multicenter cohort study to assess HRQoL, fatigue, anxiety, and depression in long-term advanced melanoma survivors treated with ipilimumab initially without subsequent systemic treatment.
Patients and methods
Participants and procedures
This cohort study was conducted in 15 hospitals in the Netherlands and Belgium. Survivors eligible for this study were ≥18 years of age, had survived at least 2 years following last administration of ipilimumab for advanced melanoma (unresectable stage III/IV) and were not diagnosed with recurrent systemic disease at the time of inclusion. Survivors were excluded if they required subsequent systemic anticancer treatment after initial ipilimumab treatment.
Eligible survivors were informed about the study by their treating medical specialist. Survivors willing to participate were asked to provide a signed and dated informed consent form. Questionnaires were mailed to survivors between February 2017 and June 2018. The survivor population was divided into two groups based on time since completion of ipilimumab treatment: 24–36 and ≥36 months post-ipilimumab treatment. Based on previous studies, a threshold of 36 months was used to compare a population at risk of recurrence with a population that might be considered to be cured [Citation9]. The study was approved by the institutional review board and meets the institutional review board standards.
A population of controls was recruited from the ‘Patient Reported Outcomes Following Initial treatment and Long-term Evaluation of Survivorship’ registry. Description of the data collection is provided elsewhere [Citation20]. The control population was asked to complete the same questionnaires as the survivor population apart from the melanoma-specific questionnaire. In total, 2508 (70%) members of the general population completed these questionnaires of which 226 subjects had a history of cancer (1%) and thus were not eligible as control for our study. From the 2282 available controls, we selected 265 that were individually matched to survivors based on year of birth, gender, and educational status.
Primary and secondary outcome measurements
Health-related quality of life (HRQoL) of long-time surviving advanced melanoma survivors was measured with the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30). The EORTC QLQ-C30 is a cancer-specific, 30-item questionnaire with strong validity [Citation21,Citation22]. The questionnaire is composed of five functional domains (physical, emotional, role, cognitive, and social functioning), nine symptomatic domains (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial impact), and one domain of global health and quality of life (QoL). A higher score on the functional domains and QoL indicates better functioning, while higher scores on the symptom domains indicate worse symptoms. A linear transformation was used to standardize the raw scores, so each domain was scored from 0 to 100. The guideline of Cocks et al. [Citation23] was used for the clinical interpretation of the differences in EORTC QLQ-C30 scores between the survivor and control population and within the survivor population. The clinical relevance of these differences was evaluated using domain-specific thresholds (eTable 1), in contrast to the fixed 10-point change as presented earlier [Citation24]. Small to large differences were defined as clinically relevant.
Fatigue was assessed with the Multidimensional Fatigue Inventory (MFI), a self-reported and validated instrument [Citation25] that was developed to assess fatigue in cancer patients. The MFI consists of 20 items in five domains (general, physical, and mental fatigue, reduced motivation, and activity), with higher scores indicating more fatigue. A two-point difference was indicated as a minimal clinical relevant difference of the MFI [Citation26]. The visual analog scale was used to record respondent’s self-rated fatigue on a 10-point Likert scale.
Anxiety and depression were assessed with the Hospital Anxiety and Depression Scale (HADS) [Citation27]. The HADS is a well-validated scale that includes seven questions on anxiety and depression respectively, a higher score indicating more anxiety and depression [Citation28]. Clinical level of anxiety or depressive symptoms was indicated with a score of ≥8 (mild to severe disorder) on each subscale [Citation29].
The melanoma subscale of the Functional Assessment of Cancer Therapy-Melanoma questionnaire (FACT-M) was used as a melanoma-specific questionnaire which has been validated to assess HRQoL for patients with all stages of melanoma [Citation30]. A higher score indicates a better QoL.
Demographic, clinical data and comorbidities
Sociodemographic data (age, education, and marital status) were obtained by five questions. Clinical data (diagnosis, stage of disease, and treatment modalities received) were obtained from the medical records. Self-reported comorbidities were assessed with the Self-administered Comorbidity Questionnaire (SCQ), a generic questionnaire with 14 common medical conditions [Citation31].
Assessments
All survivors received the survey (which contains all questionnaires mentioned above) at least 2 years post ipilimumab treatment. Survivors with no evidence of disease with a FU ≥36 months received one survey, survivors with a FU <36 months received follow-up surveys 12 and 24 months after the first one. The control population received the sociodemographic questions, the EORTC QLQ-C30, the MFI, the HADS, and SCQ only once.
Statistical analyses
Differences in EORTC QLQ-C30 and MFI scores between survivors and controls were estimated and adjusted for age, gender, education (primary/high school/vocational vs. college/university), and marital status (not partnered vs. partnered) using generalized estimating equations with clustering together matched individuals. Spearman’s correlation coefficients were used to assess associations between functioning and symptom burden, EORTC QLQ-C30 scores and cumulative ipilimumab dose, and MFI scores and demographic characteristics. Mann–Whitney or Kruskal–Wallis tests were used to compare EORTC QLQ-C30 scores between clinical, treatment characteristics and HADS scores. The Mann–Whitney test was also used for comparing HRQoL outcomes between survivors with vs. without brain metastases, survivors with a follow-up <36 vs. ≥36 months post ipilimumab treatment and survivors with ≥2 vs. without comorbidities. Sign test was used for comparison of questionnaire evaluated at two-time points. Missing items from the EORTC QLQ-C30 were imputed according to EORTC guidelines. Of all questionnaires, the scale score was set to missing, if fewer than half of the items on a given scale were answered. Since our research was a hypothesis-generating research, none of the p values were corrected for multiple testing.
Results
Participant characteristics
Of 106 invited advanced melanoma survivors, 91 (86%) returned the survey. Two survivors were excluded because they had received subsequent systemic treatment after ipilimumab treatment. Therefore, 89 survivors in total were included in the analyses. The mean age of survivors at the time of the first assessment was 64 (range 23–87, SD 13.6) years. Most survivors were partnered (70%) and highly educated; 28% had a college/university degree and only 3% of the survivors had primary education. For each survivor, at least one control was identified with the same year of birth, gender, and education level. The maximum number of controls per survivor was 5. For 29, 10, 12, 10, and 28 survivors we found 1, 2, 3, 4, and 5 controls, respectively, which resulted in a total of 265 controls included in the analyses. As a result of the individual matching, sociodemographic characteristics were comparable between the survivor and control population (). All survivors were diagnosed with unresectable stage III/IV melanoma and 13 (15%) survivors had brain metastasis at study entry. Most survivors (61%) received systemic therapy prior to ipilimumab treatment. The median time from last ipilimumab administration to the first survey was 39 (range 17–121, SD 20.8) months. Clinical and treatment characteristics are presented in .
Table 1. Sociodemographic characteristics of the survivor and control population.
Table 2. Clinical and treatment characteristics of the survivor population.
EORTC QLQ-C30 outcomes
The mean EORTC QLQ-C30 scores of physical (83.7 vs. 89.8; difference (diff) = −5.80, p=.005), role (83.5 vs. 90; diff = −5.97, p=.02), cognitive (83.7 vs. 91.9; diff = −8.05, p=.001), and social functioning (86.5 vs. 95.1; diff = −8.49, p= <.001) were significantly lower for survivors than for controls and these differences were of small clinical relevance (6–8 points) ( and ). Survivors reported a higher global QoL than the control population (80.3 vs. 78.1; p=.25), however, this was neither statistically significant nor clinically relevant.
Figure 1. Mean scores (unadjusted) of the QLQ-C30 (functional and symptom scores) of the survivor and control population.
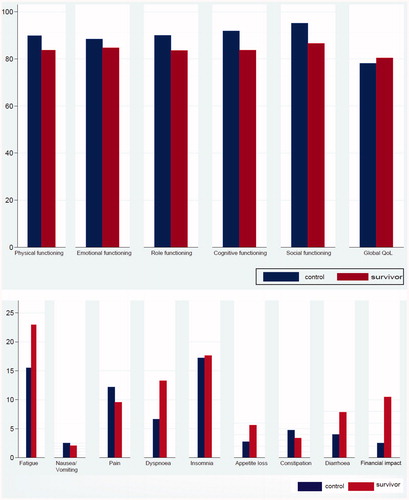
Table 3. Survivor-control differences in mean EORTC QLQ-C30 scores.
The mean EORTC QLQ-C30 symptom scores of fatigue (23.0 vs. 15.5; diff =7.48, p=.004), dyspnea (13.3 vs. 6.7; diff = 6.47, p=.02), diarrhea (7.9 vs. 4.0, diff = 3.78, p=.04), and financial impact (10.5 vs. 2.5; diff = 8.07, p=.001) were significantly higher in survivors than in controls and of small clinical relevance (4–8 points).
Survivors with ≥2 comorbidities had worse physical (diff = −16.1, p=.0001), emotional (diff = −8.5, p=.12), role (diff = −15.6, p=.002) and social (diff = −8.4, p=.05) functioning scores and global QoL (diff = −13.3 p=.002), and more complaints of fatigue (diff = 14.6, p=.005), dyspnea (diff = 10.1, p=.06), insomnia (diff = 11.7, p=.12), appetite loss (diff = 9.0, p=.007), and diarrhea (diff = 10.3, p=.17), compared to survivors without comorbidities. These differences were of small to medium clinical relevance (8–16 points) (eTable 2).
There was no correlation between EORTC QLQ-C30 scores and cumulative ipilimumab dose. Survivors with brain metastasis scored lower on cognitive (diff = −7.95, p=.27), social functioning (diff = −9.73, p=.13) and QoL (diff = −5.49, p=.27), lower on symptom burden of diarrhea (diff = −9.21, p=.07), and higher on financial impact (diff = 8.74, p=.06), compared to survivors without brain metastasis. These differences were of small to medium clinical relevance (eTable 3).
Social functioning (diff = 10.32, p=.03), global QoL (diff = 7.8, p=.10), and financial impact (diff = 8.45, p=.07) scores were higher and fatigue scores were lower (diff = −6.96, p=.25) among survivors with a FU ≥36 months in comparison to survivors with a FU <36 months. All these differences were indicated as of small clinical relevance but only the difference in social functioning was statistically significant (eTable 4).
Twenty-seven out of 40 (68%) survivors without subsequent systemic treatment and with FU < 36 months at study entry completed a second survey 12 months after the first one. From those survivors, six survivors dropped out from analyses because of receiving systemic treatment (n = 1), not willing to participate in FU (n = 3) or death (n = 2). In 7 other survivors, the second assessment was not available at data lock. The median time between the first and second assessment was 13 (range, 6–22) months. The mean EORTC QLQ-C30 scores for social functioning were higher, and symptom scores of dyspnea and diarrhea were lower at the second assessment. These differences were not statistically significant and only social functioning (8 points) was clinically relevant (eFigure 1).
Fatigue measured by the MFI
The mean mental fatigue score was significantly higher (diff = 1.0, p=.03) in survivors compared with controls but not indicated as clinically relevant (). General and mental fatigue scores decreased with increasing age (p=.02 and p=.006, respectively) and increased with higher education (p=.02 and p=.01, respectively). Physical fatigue scores decreased with higher education (p=.02) and among partnered in comparison to not partnered participants (p=.02).
Table 4. Survivor-control differences in Multidimensional Fatigue Inventory (MFI).
FACT-M and HADS
The mean FACT-M melanoma subscale score was 139.9 (SD 19.9). Of the survivors, 44% reported to be limited in social activities and 21% isolated themselves because of their condition (eTable 5). Moreover, 56% of the survivors reported memory and concentration problems. Surgery-site pain and swelling were reported in 21 and 19% of the survivors, respectively, and in the latter group, this symptom kept them from doing things they wanted to do.
The mean HADS-Anxiety and HADS-depression scores were 4.21 (SD 4.1) and 3.75 (SD 3.6), respectively. Sixteen (18%) and eleven survivors (12%) had clinical levels of anxiety and depression (≥8), respectively. Survivors with clinical symptoms of anxiety or depression had lower EORTC QLQ-C30 functioning scores, lower global QoL, higher symptom burden of fatigue, dyspnea, insomnia, appetite loss, diarrhea, and financial difficulties in comparison to survivors without symptoms. Most of these differences were indicated as clinically relevant (eTables 6 and 7).
Discussion
Ipilimumab was the first treatment that actually prolonged overall survival, which resulted in a subset of advanced melanoma patients in long term survival. Our work shows that long-term advanced melanoma survivors have a poorer HRQoL than population controls of the same age, gender, and education level. In particular, survivors have a lower level of functioning (physical, role, cognitive, and social), a higher symptom burden (fatigue, dyspnea, and diarrhea), and more financial difficulties indicated as clinically relevant. These findings reflect that survivors are still suffering from their diagnosis of advanced melanoma and the treatment trajectory. Previously, physical, psychosocial, and cognitive problems have been reported and associated with reduced HRQoL levels in cancer survivor populations [Citation32–34]. In this study, 61% received systemic non-ICI treatment and almost all survivors had surgery prior to ipilimumab. Moreover, survivors were compared with a control population not having cancer because of the absence of HRQoL data of advanced melanoma survivors without ICI treatment. Consequently, the nature of this study did not allow us to assess causality between reduced HRQoL and ipilimumab as a separate factor.
Two other studies have evaluated patient-reported outcomes of advanced melanoma patients treated with ICI (ipilimumab and/or nivolumab or pembrolizumab). Using a non-cancer-specific measurement, O’Reilly et al. [Citation35] found that 73 patients had lower physical, social functioning, and general health levels, compared with an unmatched, normative population after a median follow-up of 25 months. These HRQoL results were established in 30% of patients on various ICI treatments. Lai-Kwon et al. [Citation36] found that long-term responders to ICI (n = 69) experienced chronic treatment toxicities and psychological morbidity. Fifty-seven percent of the patients were receiving active treatment. However, they did not assess cancer-specific HRQoL. Although outcomes of the two studies are in line with this study results, the patient populations were heterogeneous. Follow-up was short, controls were not matched and ICI treatments were variable. Most notably, significant proportions of patients were on active ICI treatment. As a result of the significant differences in methodology, study population and endpoints, a formal comparison between the three studies would not be appropriate. In a cross-sectional study in 90 advanced melanoma patients after completion of ICI treatment, fatigue was the most commonly reported symptom [Citation37]. This study result is in line with our results with higher symptom burden of fatigue in the survivor population in comparison to the control population.
In comparison with a population of matched controls, we found that long-term advanced melanoma survivors showed overall worse functioning scores and more symptom burden, but surprisingly no difference on global QoL. The absence of differences in global QoL may simple reflect a response shift. Response shift refers to a change in patients’ internal standard, values, and conceptualization of QoL [Citation38] and has been reported previously in other cancer survivor populations [Citation39]. Advanced melanoma survivors have faced a life-threatening diagnosis and may have adapted to their new health situation and thereby experience a change in their frame of reference. However, the experience of a life-threatening diagnosis may also evoke a positive impact such as personal growth or an increased sense of meaning or purpose [Citation40].
Fifty-six percent of the survivors reported memory and concentration complaints. However, cancer-related cognitive impairment (CRCI) is frequently reported and seems to be associated with cancer and all components of cancer treatment [Citation41]. Despite the fact that there is limited research describing the effects of ICI on the brain, given the highly regulated immune response in the brain, it is expected that all the treatment modalities and cancer itself might be associated with CRCI [Citation42–44].
In this study, 18 and 12% of the advanced melanoma survivor population had clinical levels of anxiety and depression, respectively. These results are in line with the results of earlier studies in other cancer survivor populations [Citation45,Citation46]. Mitchell et al. found a prevalence of 17.9% for anxiety and 11.6% for depression in a meta-analysis, comparing depression and anxiety in long-term cancer survivors (≥2 years after ‘any’ cancer diagnosis) [Citation47].
Limitations of this study include the unavailability of HRQoL baseline values prior to ipilimumab and the potential participation bias that is created by the sole inclusion of advanced melanoma survivors with sufficient understanding of the Dutch language. Noteworthy is the high percentage of well-educated survivors in this study for which several possible explanations can be hypothesized. Previous studies have reported that a higher socioeconomic status (SES) is related to a higher risk of melanoma [Citation48,Citation49]. Another reason could be that those with higher SES are more likely to actively seek early access to advanced treatments, including ICI [Citation50]. These findings stress the relevance of control populations matched for educational status in studies assessing HRQoL, since education is positively associated with HRQoL [Citation51]. Strengths of this study include its homogenous survivor population not on active systemic treatment, high response rate, long-term follow-up, and an age-, gender, and education-matched control population.
In conclusion, in this cohort study, new insights are provided into the potential physical, psychological, and social morbidity of this new and growing group of cancer survivors. Knowledge of the challenges that long-term ICI-treated advanced melanoma survivors face, may help to develop tailored interventions for the individual healthcare needs of survivors, and contribute to the development of appropriate and adequate survivorship care. With the introduction of novel treatments in the recent years, the number of advanced melanoma survivors is expected to grow further, and insight in the individual healthcare needs becomes even more relevant.
Author contributors
AHB, KJJ, CUB designed the study and wrote the study protocol. AR, MJB, AJE, GAH, JWBG, MJBA, EK, AJT, DP, GV, AAV, KMPS, EAR, BN and CUB recruited patients and collected data. AHB and LVP coordinated the study. AHB and KJ did the statistical analyses. AHB, LVP and CUB wrote the first draft of the manuscript. All authors interpreted the data, reviewed the manuscript, and approved the final version.
Supplemental Material
Download MS Word (96.8 KB)Supplemental Material
Download MS Word (53.8 KB)Acknowledgments
The authors would like to thank Dr. M. Hauptmann, Dr. W.E. Fiets, Dr. F.W. van den Berkmortel, Ms. S.H. Janssen (MSc), and Ms. C. Fokkema for their valuable contributions to this study.
Disclosure statement
A.H. Boekhout received a research grant from Bristol-Myers Squibb for this study. A. Rogiers: Bristol-Myers Squibb and Merck Sharp & Dome (consulting and advisory board). M. Boers-Sonderen: Bristol-Myers Squibb, Pierre Fabre, and Roch (advisory board). A.J.M. van den Eertwegh: Sanofi, Bristol-Myers Squibb and Roche (study grant); MSD Oncology, Roche, Pfizer, and Sanofi (travel expenses); Bristol-Myers Squibb (honoraria); Bristol-Myers Squibb, MSD Oncology, Amgen, Roche, Novartis, Sanofi, Pfizer, Ipsen, and Merck (advisory board). G.A. Hospers: Bristol-Myers Squibb, Amgen, Roche, Pfizer, Novartis, MSD (consulting and advisory board) and received research grants from Bristol-Myers Squibb and Seerave. J.W.B. de Groot: Bristol-Myers Squibb, MSD Oncology, Novartis, Pierre Fabre and Servier (consulting and advisory board). M.J.B. Aarts: Bristol-Myers Squibb, Merck Sharp & Dome, Pfizer, Pierre Fabre, Astellas, Ipsen and Novartis (consulting). K.P.M. Suijkerbuijk reports personal fees as a consult advisor (paid to institution) advisory role: Roche, Novartis, MSD, BMS, Pierre Fabre (all paid to institution). H.W. Kapiteijn: Amgen, Bristol-Myers Squibb, Novartis, Roche, Merck, Pierre-Fabre, EISAI, Bayer and Genzyme-Sanofi (consulting and advisory board); and received a research grant from Bristol-Myers Squibb. A.A.M. van der Veldt: Bayer (travel expenses) and Bristol-Myers Squibb, Merck Sharp & Dome, Roche, Novartis, Pierre Fabre, Pfizer, Sanofi, Eisai and Ipsen (consulting and advisory board). E.A. Rozeman: Merck Sharp & Dome and NanoString (travel expenses). K.J. Jansen is working at Bristol-Myers Squibb. B. Neyns: Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, and Roche (honoraria), and Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, Roche, Speakers’ Bureau-Novartis (consulting and advisory), and Amgen, Bristol-Myers Squibb, Merck Sharp & Dome, Novartis, and Roche (travel expenses). C.U. Blank reports personal fees as a consultant advisor (paid to the institution) or travel support. All other authors declare no competing interests (Vreugdenhil, Kasia, Djura, and ten Tije).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356.
- Long GV, Eroglu Z, Infante J, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2018;36(7):667–673.
- Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492.
- Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36(17):1668–1674.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835.
- Eggermont AMM, Robert C, Suciu S. Adjuvant pembrolizumab in resected stage III melanoma. N Engl J Med. 2018;379(6):593–595.
- Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894.
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168.
- Revicki DA, van den Eertwegh AJ, Lorigan P, et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes. 2012;10(1):66.
- Schadendorf D, Larkin J, Wolchok J, et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur J Cancer. 2017;82:80–91.
- Shuk E, Shoushtari AN, Luke J, et al. Patient perspectives on ipilimumab across the melanoma treatment trajectory. Support Care Cancer. 2017;25(7):2155–2167.
- Cheung WY, White MK, Bayliss MS, et al. Patient-reported treatment-related symptom burden for patients with advanced melanoma in Canada. Support Care Cancer. 2018;26(6):1985–1991.
- Cheung WY, Bayliss MS, White MK, et al. Humanistic burden of disease for patients with advanced melanoma in Canada. Support Care Cancer. 2018;26(6):1985–1991.
- Rogiers AD, Ben Salama L, et al. Psychosocial outcome and health-related quality of life (HRQoL) in advanced melanoma survivors. Orlando (FL): Cancer Survivorship Symposium Orlando; 2018.
- Rogiers A, Boekhout A, Schwarze JK, et al. Long-term survival, quality of life, and psychosocial outcomes in advanced melanoma patients treated with immune checkpoint inhibitors. J Oncol. 2019;2019:5269062.
- Schadendorf DL, Wolchok J, et al. Patient-reported quality of life of patients with advanced Melnoma in a phase III study of nivolumab with or without ipilimumab versus ipilimumab alone: 4-year data from check mate 067. American Society of Clinical Oncology, Annual Meeting; Orleando; May 31–June 4, 2019.
- van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–2194.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Hjermstad MJ, Fossa SD, Bjordal K, et al. Test/retest study of the European organization for research and treatment of cancer core quality-of-life questionnaire. J Clin Oncol. 1995;13(5):1249–1254.
- Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for determination of sample size and interpretation of the European organization for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol. 2011;29(1):89–96.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144.
- Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosomat Res. 1995;39(3):315–325.
- Purcell A, Fleming J, Bennett S, et al. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer. 2010;18(3):307–315.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Spinhoven P, Ormel J, Sloekers PP, et al. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370.
- Castelli L, Binaschi L, Caldera P, et al. Fast screening of depression in cancer patients: the effectiveness of the HADS. Eur J Cancer Care. 2011;20(4):528–533.
- Cormier JN, Ross MI, Gershenwald JE, et al. Prospective assessment of the reliability, validity, and sensitivity to change of the functional assessment of cancer therapy-melanoma questionnaire. Cancer. 2008;112(10):2249–2257.
- Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163.
- Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(11):2124–2130.
- Koch L, Jansen L, Brenner H, et al. Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors-a systematic review of quantitative studies. Psychooncology. 2013;22(1):1–11.
- de Ligt KM, Heins M, Verloop J, et al. Patient-reported health problems and healthcare use after treatment for early-stage breast cancer. Breast. 2019;46:4–11.
- O’Reilly A, Hughes P, Mann J, et al. An immunotherapy survivor population: health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support Care Cancer. 2020;28(2):561–570.
- Lai-Kwon J, Khoo C, Lo S, et al. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J Cancer Surviv. 2019;13(4):503–511.
- Mamoor M, Postow MA, Lavery JA, et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J Immunother Cancer. 2020;8(1):e000260.
- Westerman MJ, Hak T, Sprangers MA, et al. Listen to their answers! Response behaviour in the measurement of physical and role functioning. Qual Life Res. 2008;17(4):549–558.
- Friedrich M, Zenger M, Hinz A. Response shift effects of quality of life assessments in breast cancer survivors. Eur J Cancer Care. 2019;28(2):e12979.
- Bower JE, Meyerowitz BE, Desmond KA, et al. Perceptions of positive meaning and vulnerability following breast cancer: predictors and outcomes among long-term breast cancer survivors. Ann Behav Med. 2005;29(3):236–245.
- Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris international cognition and cancer task force symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830–841.
- McGinnis GJ, Friedman D, Young KH, et al. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017;8(6):9155–9173.
- McGinnis GJ, Raber J. CNS side effects of immune checkpoint inhibitors: preclinical models, genetics and multimodality therapy. Immunotherapy. 2017;9(11):929–941.
- Bartels F, Stronisch T, Farmer K, et al. Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann Oncol. 2019;30(5):823–829.
- Beutel ME, Fischbeck S, Binder H, et al. Depression, anxiety and quality of life in long-term survivors of malignant melanoma: a register-based cohort study. PLoS One. 2015;10(1):e0116440.
- Gotze H, Friedrich M, Taubenheim S, et al. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer. 2020;28(1):211–220.
- Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14(8):721–732.
- van der Aa MA, de Vries E, Hoekstra HJ, et al. Sociodemographic factors and incidence of melanoma in the Netherlands, 1994–2005. Eur J Cancer. 2011;47(7):1056–1060.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004–2006. J Am Acad Dermatol. 2011;65(1):S58–S68.
- Hillen MA, Medendorp NM, Daams JG, et al. Patient-driven second opinions in oncology: a systematic review. Oncologist. 2017;22(10):1197–1211.
- Kim JH, Park EC. Impact of socioeconomic status and subjective social class on overall and health-related quality of life. BMC Public Health. 2015;15(1):783.
