Abstract
Background
There are limited data on the role of chemotherapy in patients with small cell lung cancer (SCLC) and poor performance status (PS).
Methods
This was a retrospective analysis of a prospective observational study in patients with SCLC and PS 3 or 4. We recorded the initial therapy, symptom improvement, response rate, overall survival (OS), and the impact of various factors on OS.
Results
From June 2010 to August 2019, we enrolled 234 patients; 185 (79%) with PS 3 and 49 (21%) PS 4. Initial therapy was best supportive care (BSC) in 49 patients (21%), standard full dose chemotherapy in 31 (13%), and attenuated chemotherapy in 154 (66%). In 89% patients treated with attenuated chemotherapy, symptom-relief occurred at a median of 3 days (IQR, 1–7). Grade 3 and higher toxicities developed in 60% patients treated with initial attenuated chemotherapy, commonly hyponatremia in 39%, neutropenia in 16%, anemia in 11%, and infection in 10%. Grade 3 and higher toxicities as a result of standard chemotherapy occurred in 89% patients treated with upfront standard full dose chemotherapy compared to 69% of patients who received initial attenuated chemotherapy with subsequent treatment escalation. Overall, there were 6 (2.6%) toxic deaths. The response rate to chemotherapy was 77%. The median OS of the patients who received any chemotherapy was significantly longer at 6 months (95% CI, 4.8–7.2) compared to 1 month (95% CI, 0.4–1.6 months) in patients who were managed with BSC, p < 0.001; hazard ratio, 0.39 (95% CI, 0.27–0.56). The disease stage, lactate dehydrogenase level, and receipt of chemotherapy significantly impacted survival.
Conclusion
Chemotherapy prolongs survival in patients with SCLC and poor PS. Administering an initial attenuated chemotherapy regimen followed by standard full-dose chemotherapy when the PS improves may lower toxicity and improve tolerance.
Introduction
Poor performance status (PS), i.e. PS 3 or 4 is not uncommon in patients with small cell lung cancer (SCLC), but management has not been clearly defined. All the landmark trials that established the role of chemotherapy in SCLC were conducted in patients with good PS (Eastern Cooperative Oncology Group or ECOG PS 0 to 2) [Citation1–3]. There are practically no studies that systematically evaluate the risk–benefit ratio of chemotherapy in patients with SCLC and poor PS. A retrospective analysis from Brazil in 40 patients with SCLC and PS of 3 or 4 who were treated with chemotherapy concluded that the median overall survival (OS) was poor (64 days for patients with ECOG PS 3 and 7 days for patients with ECOG PS 4) with many early deaths. The authors recommended more studies to determine the best treatment for these patients and to minimize early treatment-related mortality [Citation4].
The National Comprehensive Cancer Network (NCCN) guidelines recommend the use of systemic chemotherapy in patients with SCLC with PS 3 or 4, if the poor PS is because of the disease, rather than secondary to comorbidities [Citation5], however to the best of our knowledge, there is no randomized study that proves that chemotherapy prolongs survival in patients with SCLC and poor PS. We therefore aimed to evaluate the optimal treatment in this poor prognosis group of patients.
Material and methods
Trial design and eligibility criteria
This was a retrospective analysis of an observational study conducted in the department of medical oncology at the Tata Memorial Hospital (TMH) in Mumbai, India. From June 2010 onwards, we prospectively maintained a database of patients with SCLC and poor PS. Eligibility criteria for inclusion were a histologic or cytologic diagnosis of SCLC, ECOG PS of 3 or 4 and no prior administration of chemotherapy. The database was maintained in Microsoft Excel format. The details entered included the patient demographics, disease-related details, investigations, treatment planned, treatment delivered, symptoms, toxicity, relapse, and survival information.
The study was approved by the Institutional Ethics Committee (IEC) of TMH. As this was a retrospective analysis, the requirement of obtaining informed consent was waived by the IEC. The study was registered with the Clinical Trials Registry of India (CTRI/2017/12/010918). The study was conducted according to the principles laid down by the International Conference on Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki.
Study procedures
This was an observational study; we recorded the patient-, treatment-, and outcome-related details in the database, but no changes were made in the management. Patients were evaluated in the multidisciplinary thoracic oncology disease management tumor board; investigations and therapy were decided by the treating team. All patients underwent baseline radiologic imaging using CT scan/PET-CT scan and MRI/CT brain (optional). At our institution, patients with limited stage disease undergo a PET-CT scan to evaluate for the presence of distant metastases. Apart from this, some patients are referred to our institution from outside, with a PET-CT scan already done. An MRI brain is done in patients in whom there is a suspicion of brain metastases, either on history or examination. The decision regarding the type of therapy to be offered to the patient was made by the treating physician. The treating physician also decided whether to use granulocyte colony stimulating factors (GCSF), antibiotics and other supportive care measures as well as whether to administer radiotherapy. Patients started on chemotherapy were advised restaging CT scans every two to three months. The choice of restaging scan was predominantly a contrast enhanced CT thorax, but was dependent on the treating physician. Repeat radiologic imaging was not performed to confirm the responses. The imaging scans were read by experienced oncologic radiologists at TMH, as per the institutional practice.
At each visit of the patient to the hospital, the database was updated. If the patient did not return to the hospital for follow-up, we attempted to contact the patient telephonically. Patients who could not be contacted for over six months were considered lost-to-follow-up. The database was locked on 26th Dec 2019.
Study endpoints
The goals of the study included:
To determine the percentage of patients who had an improvement in disease-attributable symptoms: Patients were asked if they had an improvement in their symptoms (binary question, yes or no response). We did not use a prespecified list of symptoms. Patients were asked whether they had any symptoms at the time of presentation, and if so, whether the symptoms were better. If the response was yes, they were asked the date that they first noticed the improvement, and to quantify, if possible, the degree of improvement in percentage points, considering the severity of the baseline presenting symptom to be 100%.
To determine the type of therapy offered to patients with SCLC with poor PS, categorized as best supportive care (BSC), attenuated chemotherapy or standard chemotherapy. We recorded the number of patients who were initially offered an attenuated chemotherapy regimen, and the number of patients who were able to receive subsequent standard chemotherapy. Standard chemotherapy was defined as full dose chemotherapy, as per the general practice followed in the hospital, the evidence-based guidelines of TMH [Citation6] and NCCN guidelines [Citation5].
To evaluate the toxicity of chemotherapy in patients with SCLC with poor PS. Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.
To determine the response rate to first line chemotherapy. Response rate was calculated according to the new Response Evaluation Criteria in Solid Tumors: revised RECIST guideline, version 1.1. Responses were not confirmed as the primary endpoint of the study was symptom improvement, and this was an observational study.
To determine the OS of patients with SCLC and poor PS. OS was calculated from the date of diagnosis to the date of death from any cause. Patients who were lost to follow-up were censored on the date that they were last known to be alive.
To determine if there were any factors that impacted OS. The factors assessed included age (analyzed as a continuous variable), baseline PS (3 versus 4), presence or absence of brain metastases, lactate dehydrogenase or LDH (normal versus elevated), comorbidities (two or more versus none or single [comorbidity assessment was from the medical history; we did not use a validated tool to assess the comorbidities]), deranged organ function at baseline (either liver or renal dysfunction ≥2 × upper limit of normal, versus normal values ranging to <2× upper limit of normal values considering serum creatinine, calculated glomerular filtration rate, transaminases and serum bilirubin) and receipt of any dose of chemotherapy (yes versus no). Results of laboratory tests that were outside the range of the normal reference values established by the hospital laboratory were considered abnormal.
Statistics
Sample size
Since this was an observational study with a retrospective analysis, we did not initially calculate a sample size. However, the IEC requested us to calculate an approximate sample size. In the literature, there were scant data on the use of chemotherapy versus BSC in patients with SCLC and poor PS. Giordano et al. reported their experience with the use of chemotherapy in patients with SCLC with severely deranged organ function, i.e. creatinine ≥3 mg/dl, bilirubin ≥3 mg/dl or platelet count ≤50 × 106/ml. The median OS in the patients who received chemotherapy was 150 days, compared to 10 days in those who received BSC only with no chemotherapy [Citation7]. Extrapolating this to our study, we expected the median OS of our patients with SCLC and poor PS who received chemotherapy to be in the range of 5 months. Thus, with an expected 5-month survival of 50% and a confidence interval of ±6%, we required approximately 250 patients to be included in the study.
Statistical analysis
Data were entered into the Statistical Package for the Social Sciences (SPSS), version 25 and RStudio Version 1.2.5019. For patient demographics and baseline characteristics, descriptive statistics have been used. To compare the groups of patients who received BSC, initial attenuated chemotherapy and standard full-dose chemotherapy, Chi-square test and ANOVA test were used. The toxicity data have been presented with absolute numbers and simple percentages. The response rate was calculated using simple percentages. The survival analysis was done by the Kaplan–Meier method [Citation8]. To evaluate the factors that affected survival, log rank test and Cox proportional hazard model were used [Citation9,Citation10].
Results
Between June 2010 and August 2019, we enrolled 234 patients. The flowchart of patient recruitment and management is provided in . There were 185 (79%) patients with SCLC and PS 3; 49 (21%) patients had a PS of 4. Demographic details are provided in . Baseline staging was done with CT scans in 171 patients (73.1%) and with PET-CT scans in 63 (26.9%). A paraneoplastic syndrome was present in 11 patients (4.7%) at presentation, including the syndrome of inappropriate ADH secretion in 7, polymyositis-1, dermatomyositis-1, Cushing’s syndrome-1, and Eaton Lambert syndrome-1. Spontaneous tumor lysis syndrome was noted in 26 patients (11.1%).
Figure 1. The flowchart of patients evaluated and enrolled on the small cell lung cancer poor performance status observational study.
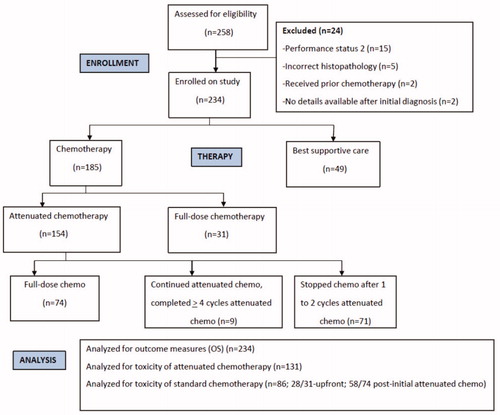
Table 1. Demographic details and baseline disease characteristics of the patients with small cell lung carcinoma who presented with poor performance status.
The details of the therapy provided are given in . Patients managed with BSC upfront appeared to have the least favorable characteristics, specifically older age, lower body weight, and poorer renal function, as compared to the patients who received attenuated or standard chemotherapy ().
Table 2. Therapy details of small cell lung cancer patients with poor performance status, i.e. ECOG PS 3 or 4.
Table 3. The demographics and baseline disease characteristics of the patients with small cell lung cancer and poor performance status, grouped according to the initial therapy they received-best supportive care, attenuated chemotherapy, and standard full-dose chemotherapy.
Of the 154 patients who received attenuated chemotherapy initially, 65 patients (42.2%) received GCSF. The median time to the start of attenuated chemotherapy was 10 days (IQR, 6–16 days) from the date the biopsy was taken. The median number of cycles of attenuated chemotherapy was 1 (IQR, 1–2). Of the 125 patients in whom the change in symptoms following the administration of chemotherapy was documented, 14 (11.2%) patients reported no improvement in symptoms; the remaining 111 (88.8%) experienced varying degrees of symptom relief. The median time from the start of attenuated chemotherapy to any degree of symptom relief was 3 days (IQR, 1–7). The toxicity of attenuated chemotherapy is detailed in Supplementary Table S1. Grade 3 and higher toxicities occurred in 78 of 131 patients (59.5%) in the attenuated chemotherapy safety set. In the 31 patients who received upfront standard chemotherapy, the median time to the start of chemotherapy was 8 days (IQR, 2–17). Grade 3 and higher toxicities occurred in 25 of 28 patients (89.3%) who received upfront standard chemotherapy and for whom toxicity details were available (Supplementary Table S2).
In the 154 patients who were started on initial attenuated chemotherapy, 74 (48.1%) patients went on to receive standard full dose chemotherapy. The median time from diagnosis to the start of standard chemotherapy was 35 days (IQR, 27–48 days). The PS at the start of standard chemotherapy was 0–2 in 52 patients (74%), and PS 3 in 10 patients (13.5%). The standard chemotherapy regimen in all patients was platinum and etoposide; in 74% patients, the platinum administered was carboplatin. In 50 patients (67.6%), GCSF was used. The median number of cycles of standard chemotherapy was 4 (IQR, 2–5). 43 patients (69.4%) experienced ≥grade 3 toxicities from standard chemotherapy (Supplementary Table S2).
In the 93 patients who underwent disease re-assessment, the response rate to chemotherapy was 77.4% (complete remission 5.4%, partial remission 72%); 9.7% had stable disease and 12.9% had progressive disease.
None of the 24 patients with limited stage disease were fit for concurrent chemoradiotherapy at the baseline assessment, given their compromised PS. The initial therapy offered to the patients with limited stage disease was BSC in 5, attenuated chemotherapy in 14 and standard full dose chemotherapy in 5 patients. Of the 14 patients who were started on initial attenuated chemotherapy, 9 had an improvement in their PS to 0–2 and subsequently, received standard full dose chemotherapy. Of the remaining five patients, two defaulted and three patients did not experience an improvement in PS, and therefore could not receive standard full dose chemotherapy. Regarding thoracic radiation, 3 (12.5%) patients were treated with concurrent chemoradiation, 4 (16.8%) received sequential curative intent chemotherapy and radiotherapy, and 17 patients (70.8%) were treated with palliative intent. The median dose of thoracic radiation in patients who were treated with radical intent was 50 Gy (IQR, 46–56), in a median of 25 fractions (IQR, 25–28) over a median of 47 days (IQR, 37–52).
The median PS at the time of completion of first line chemotherapy was 1 (IQR, 1–2). In terms of exposure to adequate systemic therapy, 58 (24.8%) patients received at least four cycles of full dose combination chemotherapy, an additional 15 (6.4%) received at least four cycles of combination chemotherapy of which at least 1 cycle was given at an attenuated dose and 150 (64.1%) received less than four cycles of combination chemotherapy.
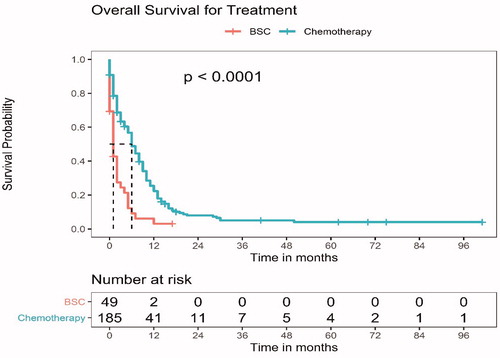
About 197 (84.2%) patients have died, 30 (12.8%) are lost to follow-up and 7 (3%) patients are alive. The median OS for the entire cohort of patients with SCLC and compromised PS was 5 months (95% CI, 3.6–6.4). The median OS for the patients who received any chemotherapy was significantly longer at 6 months (95% CI, 4.8–7.2) compared to 1 month (95% CI, 0.4–1.6) in patients who were managed with BSC, p < 0.001; hazard ratio, 0.39 (95% CI, 0.27–0.56) (). There was no significant difference in the median OS of the patients who received an initial attenuated chemotherapy regimen (median OS, 6 months, 95% CI, 4.5–7.6) and those who were started on upfront full-dose chemotherapy (median OS, 8 months, 95% CI, 5.6–10.4), p = 0.09 (Supplementary Figure S1). Patients who received at least four cycles of combination chemotherapy had a median OS of 10 months (95% CI, 6.2–13.8), while the patients who received less than 4 cycles of combination chemotherapy had a median OS of 2 months (95% CI, 1.2–2.8), p < 0.001. In all the tested subgroups, chemotherapy led to a significant prolongation of OS (Supplementary Figure S2). On multivariate analysis, the only factors that were significantly associated with a better OS were the baseline limited stage disease, normal LDH level and the receipt of chemotherapy. (Supplementary Table S3).
Figure 2. The overall survival (OS) for patients with small cell lung carcinoma and poor performance status (PS 3 or 4), who received any chemotherapy versus best supportive care (BSC). The median OS for the patients who received any chemotherapy was significantly longer at 6 months (95% CI, 4.8–7.2) compared to 1 month (95% CI, 0.4–1.6) in patients who were managed with BSC, p < 0.001; hazard ratio, 0.39 (95% CI, 0.27–0.56).
Discussion
We found that in patients with SCLC and compromised PS, i.e. PS 3 or 4 who were managed with BSC, the median OS was 1 month, compared to 6 months in patients who received any dose of chemotherapy. This was both a clinically relevant and statistically significant improvement. In patients with SCLC, chemotherapy is known to prolong survival as compared to BSC, however, these studies did not include patients with poor PS [Citation11]. To the best of our knowledge, there are no randomized studies that prove the role of chemotherapy in patients with poor PS. A similar retrospective study by Azam et al. in 246 patients with SCLC and PS 3 or 4 reported that 80 patients (33%) received chemotherapy (carboplatin + etoposide in 41 [17%], single agent carboplatin in 32 [13%], oral etoposide in 7 [3%]); the remaining 166 (67%) received BSC. Similar to the results of our study, the authors reported that the median OS was 2 months (95% CI, 1.71–2.28) in the patients managed with BSC, compared to 6 months (95% CI, 3.70–8.29) in patients who received chemotherapy; p < 0.01 [Citation12].
The Medical Research Council (MRC) Lung Cancer Working Party Group conducted two randomized trials that included patients with SCLC with PS 3 or 4; 75 patients in a trial that compared a 4-drug chemotherapy regimen to a two-drug regimen [Citation13] and 128 patients in a trial that compared intravenous to oral etoposide chemotherapy [Citation14]. Hermes et al. conducted a randomized trial in 209 patients with extensive stage SCLC, comparing irinotecan and carboplatin with oral etoposide and carboplatin; there was no upper age limit and no PS specified as part of the inclusion criteria. In the 37 patients with PS 3 or 4, the median OS was 127 days and the 1-year survival was 19% [Citation15]. Neither of these trials included a nonchemotherapy arm, thus no conclusions can be drawn regarding the role of chemotherapy in patients with SCLC and poor PS. An older retrospective study in 13 patients with SCLC and PS of 3 or 4 who received chemotherapy reported that the PS improved in 8 patients (62%); there were 2 (16%) treatment related deaths from sepsis within 1 month, and the median OS were 7.8 and 8.4 months in the patients with limited stage and extensive disease respectively [Citation16]. In another retrospective analysis of 18 patients with SCLC and PS of 3 or 4, 12 patients (67%) received chemotherapy and 6 (33%) received BSC alone. Post-chemotherapy, there was an improvement in PS in 7 patients (58%), a worsening in 3 (25%) and no change in 2 (17%). The toxicities of chemotherapy included grade 3 and higher neutropenia in 83% and febrile neutropenia in 42%; there was one treatment-related mortality. The median OS was 4.9 months, and the 6-month survival was 47.9%; the survival was comparable between patients with PS 3 or 4. Chemotherapy resulted in a significant survival benefit: the 6-month survival was 66.7% in the patients who received chemotherapy, versus 0 in the patients managed with BSC. All the patients who received BSC died within the first 5 months [Citation17].
The concern about administering chemotherapy to a patient with poor PS is excessive toxicity, i.e. the possibility of doing more harm than good. In our study, 60% of the patients with SCLC and poor PS who were treated with attenuated chemotherapy developed severe acute toxicities, while 89% of the patients treated with upfront standard chemotherapy developed severe toxicities. This is broadly comparable to the reported toxicities in first line therapy in good PS patients with extensive stage SCLC; in the Japanese trial by Noda et al. that included only patients younger than 70 years with an ECOG PS of 0 to 2, 92.2% patients treated with cisplatin and etoposide developed grades 3 or 4 neutropenia and 29.9% patients had grades 3 or 4 anemia [Citation18]. In our study, other than hyponatremia, the most common toxicities included neutropenia and infectious complications, and three of the four toxic deaths that occurred in the patients on attenuated chemotherapy were due to infections. A high index of suspicion, close monitoring of the post-chemotherapy blood counts, and a low threshold for the primary administration of growth factors and antibiotics, may help to decrease the risk of infections and improve the therapeutic index of chemotherapy in these patients. The incidence of severe toxicities from full dose chemotherapy in our study in patients who received initial attenuated chemotherapy followed by standard full dose chemotherapy was 69%. Thus, the stepwise approach of administering attenuated doses of chemotherapy upfront, to chemically debulk the tumor, decrease the tumor-related symptoms and improve the PS, followed by full dose systemic chemotherapy when the PS has improved, may lower the toxicity of therapy.
PS is a well-known prognostic factor in patients with SCLC [Citation19–21]. In our study, the response rate to first line chemotherapy in patients with SCLC and poor PS was 77% and the median OS in patients who received some form of chemotherapy was 6 months. In the literature, the expected response rate to systemic chemotherapy ranges from 70 to 90% and the expected median OS of patients with extensive stage SCLC who are treated with systemic chemotherapy ranges from 8 to 13 months [Citation22]. Thus, our cohort of patients did appear to have a shorter OS than what is described in the literature, reiterating that poor PS is a powerful prognostic marker in SCLC. Within the group of poor PS patients, a PS of 3 versus 4 did not appear to carry additional prognostic value.
Our study consisted of a relatively homogenous patient cohort, i.e. we included all patients with SCLC and PS 3 or 4, and our patient cohort represented the real-world scenario in the clinic; two patients were in the intensive care unit, on mechanical ventilation and pressor support and 6% patients had stridor with impending respiratory failure. The management of the patients in the study was decided in the multidisciplinary thoracic oncology clinic and the therapy planned was relatively uniform. The overall outcomes of the patients are consistent with the data reported in the literature. As the event rate in SCLC is high, our data are mature. Several points need to be highlighted about our patient cohort, including the demographics/clinical features and the management. Almost 10% of our patients were never-smokers, which is unusually high for an SCLC cohort; 3% of the patients in the IMpower133 study and 1.3% of the patients in the CONVERT trial were never-smokers [Citation2,Citation23]. Smoking is uncommon in India: 19% of men and 2% of women smoke tobacco [Citation24]. In our earlier studies, 55% of our patients with lung cancer and 15% of our patients with SCLC were nonsmokers [Citation25,Citation26]. Only 17% of the patients in the current study were women, which is lower than that reported from the pivotal trials: 35% of the patients in the Impower133 and 46% of the patients in the CONVERT trial were women [Citation2,Citation23]. The possible explanations include the fact that smoking is rare in Indian women, and the access to healthcare by Indian women is less than that of men. In our earlier studies, women constituted 23, 11, and 19% of the overall lung cancer, SCLC, and geriatric oncology cohorts respectively [Citation25–27]. The proportion of patients with comorbidities was also lower than expected at 45%. This may have been because our Indian patients are comparatively younger, and a significant proportion do not seek routine medical care for health maintenance. In our cohort of patients with EGFR mutant NSCLC, 49% of the patients had comorbidities [Citation28]. Our study was purely observational; the workup, management plan, supportive care, restaging, and follow-up were at the discretion of the treating physician. Our definition of OS was also different from the standard. As there was a group of patients who were treated with BSC, we took the date of diagnosis (rather than the date of start of therapy) as the start date for the OS calculation. Dosing of chemotherapy was not standard in all the patients. There was a cohort of patients who received dose reduced chemotherapy, and these patients had good clinical benefit. Standard doses of chemotherapy could be started in a significant proportion of patients.
The major drawback of our study is inherent in the type of the study; this was an observational study, rather than a randomized trial. The therapy decided was probably influenced by patient and physician biases, i.e. BSC for the sickest patients and full dose chemotherapy for the patients who were fittest; this is borne out by the differences in the demographic and disease profile between the three groups of patients (grouped according to the therapy decided at baseline). Since the poorest prognosis patients may not have received chemotherapy, the results of the study may have been skewed in favor of the patients who did receive chemotherapy. Unfortunately, a randomized controlled trial in patients with SCLC to prove the role of chemotherapy in poor PS patients may not be considered ethical, even though there is no real level 1 evidence in this setting. In the absence of data from randomized controlled trials, data from large observational studies like ours provide valuable information. It is possible that not all the patients with SCLC and compromised PS were entered in the prospective database. As we only maintained the database for the patients with SCLC who had compromised PS, we were unable to describe what proportion the PS 3–4 patients comprised of the entire pool of patients with SCLC. The symptom assessment was just a ‘yes’ or ‘no’ answer; a formal validated quality of life questionnaire was not used. Since our hospital is a referral oncology center, many patients travel from distant places to obtain management recommendations. Some of these patients return to their local hospital to receive therapy there and some do not return to our hospital. We had a 12.8% lost to follow-up rate, which is relatively high.
Conclusion
Chemotherapy prolongs survival in patients with SCLC and poor PS. A stepwise approach in which an initial attenuated dose of chemotherapy is administered followed by full dose standard chemotherapy once the patient’s PS has improved may lower toxicity and improve tolerability.
Supplemental Material
Download MS Word (13.1 KB)Supplemental Material
Download MS Word (14.9 KB)Supplemental Material
Download MS Word (13.4 KB)Supplemental Material
Download MS Word (243.7 KB)Supplemental Material
Download MS Word (205.2 KB)Supplemental Material
Download MS Word (170.2 KB)Disclosure statement
All the other authors declared no conflicts of interest.
Additional information
Funding
References
- Sundstrom S, Bremnes RM, Kaasa S, et al.; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20(24):4665–4672.
- Faivre-Finn C, Snee M, Ashcroft L, et al.; CONVERT Study Team. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125.
- Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–2535.
- Baldotto CS, Cronemberger EH, de Biasi P, et al. Palliative care in poor-performance status small cell lung cancer patients: is there a mandatory role for chemotherapy? Support Care Cancer. 2012;20(11):2721–2727.
- Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. [cited 2018 Mar 5].
- Available from: https://tmc.gov.in/tmh/PDF/Lung-Gastric.pdf. [cited 2018 Mar 5].
- Giordano KF, Jatoi A, Adjei AA, et al. Ramifications of severe organ dysfunction in newly diagnosed patients with small cell lung cancer: contemporary experience from a single institution. Lung Cancer. 2005;49(2):209–215.
- Chakraborty S. A step-wise guide to performing survival analysis. Cancer Res Stat Treat. 2018;1:41–45.
- Dessai S, Simha V, Patil V. Stepwise cox regression analysis in SPSS. Cancer Res Stat Treat. 2019;2(1):108–170.
- Dessai S, Patil V. Testing and interpreting assumptions of COX regression analysis. Cancer Res Stat Treat. 2019;2(1):108–111.
- Agra Y, Pelayo M, Sacristan M, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2003;(4):CD001990.
- Azam F, Wong H, Green JA, et al. Poor performance status small cell lung cancer: who should we treat? J Clin Oncol. 29(15). DOI:10.1200/jco.2011.29.15_suppl.e17502
- by Bleehen NM, Girling DJ, Hopwood P, et al. Medical Research Council Lung Cancer Working Party (prepared on behalf of the working party and its collaborators. Randomised trial of four-drug vs less intensive two-drug chemotherapy in the palliative treatment of patients with small-cell lung cancer (SCLC) and poor prognosis. Br J Cancer. 1996;73(3):406–413.
- Girling DJ. Comparison of oral etoposide and standard intravenous multidrug chemotherapy for small-cell lung cancer: a stopped multicentre randomised trial. Medical Research Council Lung Cancer Working Party. Lancet. 1996;348(9027):563–566.
- Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26(26):4261–4267.
- Sakuragi T, Oshita F, Nagashima S, et al. Retrospective analysis of the treatment of patients with small cell lung cancer showing poor performance status. Jpn J Clin Oncol. 1996;26(3):128–133.
- Aida Y, Nakazawa K, Shiozawa T, et al. Small-cell lung cancer treatment of newly diagnosed patients with poor performance status. Case Rep Oncol. 2019;12(2):613–620.
- Noda K, Nishiwaki Y, Kawahara M, et al.; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85–91.
- Cerny T, Blair V, Anderson H, et al. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer. 1987;39(2):146–149.
- Kawahara M, Fukuoka M, Saijo N, et al. Prognostic factors and prognostic staging system for small cell lung cancer. Jpn J Clin Oncol. 1997;27(3):158–165.
- Maestu I, Pastor M, Gómez-Codina J, et al. Pretreatment prognostic factors for survival in small-cell lung cancer: a new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol. 1997;8(6):547–553.
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now? A review. Transl Lung Cancer Res. 2016;5(1):26–38.
- Horn L, Mansfield AS, Szczęsna A, IMpower133 Study Group, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Available from: https://www.who.int/tobacco/surveillance/survey/gats/GATS_India_2016-17_FactSheet.pdf?ua=1#:∼:text=19.0%25%20of%20men%2C%202.0%25,and%2For%20smokeless%20tobacco. [cited 2020 July 30].
- Noronha V, Dikshit R, Raut N, et al. Epidemiology of lung cancer in India: focus on the differences between non-smokers and smokers: a single-centre experience. Indian J Cancer. 2012;49(1):74–81.
- Noronha V, Chougule A, Joshi A, et al. Epidermal growth factor receptor mutation in small cell lung cancer patients in an indian tertiary care oncology hospital: incidence and clinical outcome. Clin Oncol. 2016;28(5):342–343.
- Noronha V, Ramaswamy A, Dhekle R, et al. Initial experience of a geriatric oncology clinic in a tertiary cancer center in India. Cancer Res Stat Treat. 2020;3(2):208–217.
- Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Cin Oncol. 2020;38(2):124–136.
