Abstract
Introduction
A meningeal solitary fibrous tumor (SFT), also called hemangiopericytoma, is a rare mesenchymal malignancy. Due to anatomic constrains, even after macroscopic complete surgery with curative intent, the local relapse risk is still relatively high, thus increasing the risk of dedifferentiation and metastatic spread. This study aims to better define the role of postoperative radiotherapy (RT) in meningeal SFTs.
Patients and methods
A retrospective study was performed across seven sarcoma centers. Clinical information was retrieved from all adult patients with meningeal primary localized SFT treated between 1990 and 2018 with surgery alone (S) compared to those that also received postoperative RT (S + RT). Differences in treatment characteristics between subgroups were tested using independent samples t-test for continuous variables and chi-square tests for proportions. Local control (LC) and overall survival (OS) rates were calculated as time from start of treatment until progression or death from any cause. LC and OS in groups receiving S or S + RT were compared using Kaplan–Meier survival curves.
Results
Among a total of 48 patients, 7 (15%) underwent S and 41 (85%) underwent S + RT. Median FU was 65 months. LC was significantly associated with treatment. LC after S at 60 months was 60% versus 90% after S + RT (p = 0.052). Furthermore, R1 resection status was significantly associated with worse LC (HR 4.08, p = 0.038). OS was predominantly associated with the mitotic count (HR 3.10, p = 0.011).
Conclusion
This retrospective study, investigating postoperative RT in primary localized meningeal SFT patients, suggests that combining RT to surgery in the management of this patient population may reduce the risk for local failures.
Introduction
Solitary fibrous tumors (SFT) are rare mesenchymal malignancies with an overall incidence of about 0.2/100.000 per year [Citation1]. They can arise anywhere in the body, including the central nervous system/meninges, the head and neck and, most often, the chest and the extremities.
The disease was formerly called hemangiopericytoma. This name has been abandoned in the WHO classification of soft tissue and bone tumors, after observing that SFT does not arise from pericytes, whereas it is still retained in the WHO classification of CNS tumors [Citation1]. The last edition of WHO classification of CNS tumors, released in 2016 [Citation2], assigned three grades within the entity, while in the WHO classification of soft tissue and bone tumor SFT were not graded [Citation3–5].
Finally, some tumors with a histological appearance more similar to traditional SFT can also display malignant features and be assigned a WHO grade III, using the cutoff of 5 or more mitoses per 10 high-power fields. From the molecular point of view the disease is marked by the nuclear expression of STAT6 [Citation6].
Imaging features of these tumors are nonspecific, leaving a broad list of differential diagnoses. The most prominent differential diagnosis intracranially is meningioma [Citation6–11]. Assigning the correct diagnosis can be very challenging especially in more aggressive cases. Therefore, pathologic review by an expert sarcoma pathologist, with the confirmation of STAT6 immunostaining positivity, should be considered in all cases.
As for all other sarcomas, standard treatment of primary localized meningeal SFT/hemangioperycitoma is a wide surgical resection, which is typically challenging in CNS locations [Citation5,Citation12]. Inherently, the chance of local control is known to be relatively low. The potential added value of (neo-)adjuvant radiotherapy (RT) in combination with surgery, both in the setting of a gross total resection (GTR) or after less radical surgery for local control and survival remains unclear [Citation1,Citation13–21]. The outcome after definitive RT, without surgery [Citation22], as well as the role of perioperative RT in extracranial SFTs [Citation23] has recently been described by our group.
This international collaborative retrospective study aims to better define the role of postoperative RT as compared to surgery alone in meningeal SFT management.
Patients and methods
A retrospective study was performed across seven tertiary referral sarcoma centers, collecting data of primary, localized, adult meningeal SFT patients treated by surgery alone or in combination with postoperative RT, between 1990 and 2018. Eligible patients were identified by merging hospital-based registries of documented diagnoses, by a pathology search and by queering sarcoma tumor board maintained databases. Pathological diagnosis was locally reviewed by reference sarcoma pathologists and confirmed in all cases. Clinical information on demographics, treatment and follow up was retrieved from charts of all consecutive patients undergoing surgery alone and compared to those also receiving post-operative RT. In all participating centers, approval for this retrospective database analysis was obtained from the local Medical Ethics Committees (central approval METC15.1609). All patient data were anonymized. The study was performed in close collaboration with the Sarcoma Patients EuroNet. Toxicity was graded according to CTCAE 4.0. Risk assessment was performed using the original Demicco model [Citation13], because the percentage necrosis was not registered while acquiring this database [Citation24]. The here used Demicco model cumulates the scores for age (≥55 years, score 1 point), size (<5cm; 0 points, 5–10 cm; 1 point, 10–15cm; 2 points, ≥15cm 3 points) and mitotic figures per 10 high power fields (0; 0 points, 1–3; 1 point and ≥4; 2 points). A total score of 0–2 points designates a low risk profile, 3–4 points as moderate risk and 5-6 as high risk disease. The resection margins were ultimately defined by the reference neuropathologist by microscopic assessment of the resection specimen, supplemented with information supplied by the respective neurosurgeons. An R0 resection is defined as no residual tumor after surgery. After an R1 resection, the surgical margins are microscopically positive, synonymous to a gross total resection (GTR) and after an R2 resection, the surgical margins are macroscopically positive, synonymous to a subtotal resection (STR).
The absence of metastases at diagnosis, was confirmed by chest imaging in all participating patients.
Statistical analyses
Characteristics are presented as percentage, mean (±SD) or median (+ interquartile range) in case of a skewed distribution. Differences in baseline characteristics were tested using Student’s t-tests (continuous variables), chi-square tests (categorical variables) or ANOVA-tests with post-hoc Bonferroni (for >2 groups). The follow-up duration was calculated as the time between start of treatment and date of death, loss to follow-up or date of last follow-up. Cumulative incidence, 6-month-, 1-, 2-, and 5-year mortality rates were investigated and Kaplan–Meier survival curves were plotted for overall survival (OS) and local control (LC) and differences were tested with Logrank tests. Cox proportional hazards analyses (using multiple imputation with a parametric regression method with 10 replacements for variables with missing values) were performed to investigate univariate associations between patient and treatment characteristics and outcomes. p-values less than 0.05 were considered statistically significant. Data are presented as Hazard Ratios (HR) with 95% confidence intervals (CI), which can be interpreted as relative risks. Analyses were performed with SPSS 25.0.
Results
Median FU of the entire cohort was 65 months (IQR 31–202 months). With respect to management (), this cohort was subdivided into those patients treated by surgery alone (S; n = 7, 15%) and those undergoing surgery + RT (S + RT; n = 41, 85%). Mean age was 50 years and 56% were male.
Table 1. Baseline characteristics of the entire study population, stratified by surgery (S) or surgery plus radiotherapy (S + RT).
Comparing the S + RT- versus the S-group, the rate of R2 resections was not significantly different (54% versus 50%, p = 0.749) as well as the Demicco scores (61% versus 86% low risk, p = 0.396) and the tumor sizes of 5.6 cm and 4.8 cm (p = 0.39) respectively. However, in the S + RT group, the majority of patients (15 of 21, 71%) had meningeal SFTs with a mitotic count > 4, versus 25% (1/4) in the S-group with a mitotic count > 4, (p = 0.032).
The 41 patients in the S + RT group were all irradiated postoperatively to a dose of 44–73.6 Gy in fractions of 1.6–2 Gy. Median follow in this group was 58 months (IQR 39–77 months).
Local control and overall survival analyses
The benefit for local control by the addition of RT did not reach statistical significance, but the p-value was as low as 0.052 (), with local control rates of 60% (S) and 90% (S + RT) at 60 months, respectively. In univariate analyses including multiple imputation methods, the LC probability was significantly associated with the addition of RT (HR 0.25, 95% CI 0.14–0.47, p ≤ 0.001, ).
Figure 1. Local control comparing surgery alone (S) versus surgery plus perioperative radiotherapy (S + RT).
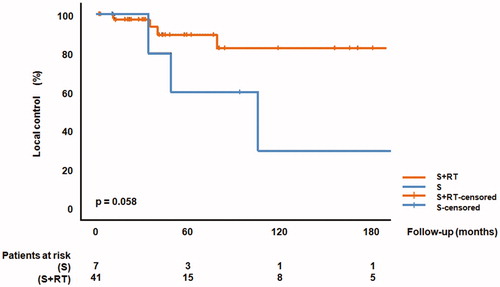
Table 2. Univariate associations with overall survival and local control.
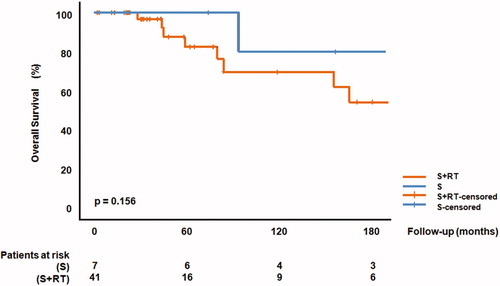
OS was not significantly different between surgery and surgery + RT (p = 0.156), with survival rates at 5 years follow up of 100% (S) versus 82% (S + RT). The 10 years survival rates were 80% (S) versus 70% (S + RT), respectively. In univariate analyses, mitotic count was predominantly associated with OS (HR 3.10, 95% CI 1.29–7.29, p = 0.011). Time to first distant relapse was not captured in this database ().
Toxicity
As far as retrospective analyses justify, toxicities and perioperative morbidities seemed to be mild and predominantly of grade 1 in both groups (e.g., skin erythema and alopecia). There was 1 patient in the S + RT group who suffered from a cerebrospinal fluid leak resolving after a temporary external ventricular drain. With relatively low numbers in both treatment groups, there was a higher incidence of both acute and late toxicities observed in the S + RT subgroup compared to the S-subgroup (50% versus 14%, p = 0.112, and 23% versus 0%, p = 0.318, respectively).
Discussion
This retrospective observational study on 48 primary, localized meningeal solitary fibrous tumor patients compared treatment by surgery alone (S) to surgery and post-operative RT (S + RT). Although the numbers are relatively small, the data presented suggest that the addition of RT may increase the LC probability (90% versus 60% at 60 months). However this did not change survival probability.
These data on previously untreated meningeal SFT patients may be clinically relevant, because surgical procedures for intracranial local failures after prior surgery alone frequently lead to serious complications and poor resection margins [Citation25]. Not only the side effects of salvage management should be considered but also the morbidity associated with a local failure in case RT is not applied. In addition, even if our series did not show a difference in OS, the occurrence of a local relapse may be associated with tumor dedifferentiation and increased risk of metastatic spread [Citation26]. Careful balancing of the risk of a local failure by the omission of RT to the risk of both acute and late radiation associated toxicities should preferentially be left to experienced multidisciplinary teams in tertiary referral centers. Considering all caveats and biases of retrospective analyses like the one here presented, the addition of RT to surgery may lead to an increase in local control. Furthermore, analyses on both acute and late toxicities may very well be subject to report biases in medical files. Due to the rarity of the disease, formal testing of this observation in prospective randomized phase III trials will never be possible.
The role of surgery, especially in the non-metastatic setting, is undisputed [Citation5,Citation27,Citation28]. As for the role of (adjuvant) RT, Ghia et al. [Citation29] have shown, that in the subgroup of patients who underwent subtotal resection, the addition of postoperative RT had a profound effect on OS. Lee and coworkers [Citation30] stated, that a LR was the main pattern of failure in patients who did not receive postoperative RT, whereas distant metastasis was the main pattern of failure in patients who received RT. Stessin et al. [Citation31] also observed, that, on multivariate analysis, postoperative RT was associated with a significantly better survival (HR = 0.269, p = 0.027), in particular for patients who underwent STR (HR = 0.088, p < 0.008). This conclusion was confirmed by Kinslow et al. [Citation32], stating that a GTR + RT was associated with significantly increased survival compared with GTR alone (HR = 0.537, p = 0.039).
Publications on perioperative RT in SFT have been summarized in . In brief, most series report on postoperative RT and have included a total 347 patients, and when patients from the current study are included, a total of 388 patients. Our LC and OS rates are consistent with these data. Furthermore, data on a cumulative of 158 patients treated by GammaKnife equipment, are also available in the literature reporting on favorable outcomes.
Table 3. Literature data on postoperative or exclusive RT in meningeal SFT.
This is a retrospective study, with all the obvious limitations thereof. Due to the relatively small sample size, the difference in local control between treatment groups showed a tendency toward statistical significance. After multiple imputation, the association between treatment and local control did show a significant benefit in favor of surgery + RT. Considering the relatively small sample size, the effect of the Kaplan–Meier might show an underestimation of the true effect. Although this is not the largest series published thus far, it is the second largest series of postoperatively irradiated, surgically treated meningeal SFT/hemangiopericytoma patients with detailed patient characteristics. Only the SEER-database study by Kinslow et al. [Citation32] is larger, but here the radiation dose, the malignancy grade (mitotic index) and local control rates are missing. Furthermore, the meta-analysis available by Ghose et al. [Citation44] on 523 patients and the National Cancer Database analysis by Trifiletti et al. [Citation45] on 991 patients, confirm our observation, that postoperative RT significantly improves OS but in also in these manuscripts detailed individual patients characteristics (specifically radiation dose) are not reported. Furthermore, the S + RT subgroup of 41 patients, being irradiated to a postoperative dose of 44–73.6 Gy in fractions of 1.6-2 Gy, does not reasonably allow any estimation of a dose-effect relationship for local control. Finally, it cannot be excluded, that longer follow up of our cohort (currently median 65 months) may reveal late recurrences.
As SFT is a rare disease with an estimated 230 cases per year in the US, it is unlikely that prospective randomized clinical trials will ever be conducted [Citation32]. Nevertheless, this study suggests that combining RT with surgery in the management of this patient population may reduce the risk for local failures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vaz Salgado MA, Soto M, Reguero ME, et al. Clinical behavior of solitary fibrous tumor: a retrospective review of 30 patients. Clin Transl Oncol. 2017;19(3):357–363.
- Louis DN, Perry A, Reifenberger G, et al. WHO classification and grading of tumours of central nervous system. In: Louis DN, Ohgaki H, Wiestler OD, et al., editors. WHO classification of tumours of the central nervous system. 4th ed., Revised. Lyon: International Agency for Research Centre; 2016. p. 12–13.
- Bertero L, Anfossi V, Osella-Abate S, et al. Pathological prognostic markers in central nervous system solitary fibrous tumour/hemangiopericytoma: evidence from a small series. PLOS One. 2018;13(9):e0203570.
- Macagno N, Vogels R, Appay R, et al. Grading of meningeal solitary fibrous tumors/hemangiopericytomas: analysis of the prognostic value of the Marseille Grading System in a cohort of 132 patients. Brain Pathol. 2019;29(1):18–27.
- Kim BS, Kim Y, Kong DS, et al. Clinical outcomes of intracranial solitary fibrous tumor and hemangiopericytoma: analysis according to the 2016 WHO classification of central nervous system tumors. J Neurosurg. 2018;129(6):1384–1396.
- Ohba S, Murayama K, Nishiyama Y, et al. Clinical and radiographic features for differentiating solitary fibrous tumor/hemangiopericytoma from meningioma. World Neurosurg. 2019;130:e383–e392.
- Vogels RJ, Vlenterie M, Versleijen-Jonkers YM, et al. Solitary fibrous tumor – clinicopathologic, immunohistochemical and molecular analysis of 28 cases. Diagn Pathol. 2014;9:224.
- Ge W, Yu DC, Chen G, et al. Clinical analysis of 47 cases of solitary fibrous tumor. Oncol Lett. 2016;12(4):2475–2480.
- Ginat DT, Bokhari A, Bhatt S, et al. Imaging features of solitary fibrous tumors. Am J Roentgenol. 2011;196(3):487–495.
- Wignall OJ, Moskovic EC, Thway K, Thomas JM. Solitary fibrous tumors of the soft tissues: review of the imaging and clinical features with histopathologic correlation. Am J Roentgenol. 2010;195(1):W55–W62.
- Wang XQ, Zhou Q, Li ST, et al. Solitary fibrous tumors of the central nervous system: clinical features and imaging findings in 22 patients. J Comput Assist Tomogr. 2013;37(5):658–665.
- Yao MJ, Ding L, Atay SM, et al. A modern reaffirmation of surgery as the optimal treatment for solitary fibrous tumors of the pleura. Ann Thorac Surg. 2019;107(3):941–946.
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–1306.
- Wushou A, Jiang YZ, Liu YR, et al. The demographic features, clinicopathologic characteristics, treatment outcome and disease-specific prognostic factors of solitary fibrous tumor: a population-based analysis. Oncotarget. 2015;6(39):41875–41883.
- DeVito N, Henderson E, Han G, et al. Clinical characteristics and outcomes for solitary fibrous tumor (SFT): a single center experience. PLOS One. 2015;10(10):e0140362.
- van Houdt WJ, Westerveld CM, Vrijenhoek JE, et al. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol. 2013;20(13):4090–4095.
- Cardillo G, Carbone L, Carleo F, et al. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg. 2009;88(5):1632–1637.
- Bisceglia M, Galliani C, Giannatempo G, et al. Solitary fibrous tumor of the central nervous system: a 15-year literature survey of 220 cases (August 1996–July 2011). Adv Anat Pathol. 2011;18(5):356–392.
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13(4):264–269.
- Soyuer S, Chang EL, Selek U, et al. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100(7):1491–1497.
- Milano MT, Singh DP, Zhang H. Thoracic malignant solitary fibrous tumors: a population-based study of survival. J Thorac Dis. 2011;3(2):99–104.
- Haas RL, Walraven I, Lecointe-Artzner E, et al. Radiation therapy as sole management for solitary fibrous tumors (SFT): a retrospective study from the global sft initiative in collaboration with the sarcoma patients EuroNet. Int J Radiat Oncol Biol Phys. 2018;101(5):1226–1233.
- Haas RL, Walraven I, Lecointe-Artzner E, et al. Extrameningeal solitary fibrous tumors; surgery alone or surgery plus perioperative radiotherapy, a retrospective study from the Global SFT Initiative in collaboration with the Sarcoma Patients EuroNet. Cancer. 2020;126(13):3002–3012. [Epub ahead of print].
- Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30(10):1433–1442.
- Wanibuchi M, Akiyama Y, Mikami T, et al. Radical removal of recurrent malignant meningeal tumors of the cavernous sinus in combination with high-flow bypass. World Neurosurg. 2015;83(4):424–430.
- Baldi GG, Stacchiotti S, Mauro V, et al. Solitary fibrous tumor of all sites: outcome of late recurrences in 14 patients. Clin Sarcoma Res. 2013;3:4.
- Pasquali S, Gronchi A, Strauss D, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: A multi-centre prognostic study. Eur J Surg Oncol. 2016;42(7):1064–1070.
- Gholami S, Cassidy MR, Kirane A, et al. Size and location are the most important risk factors for malignant behavior in resected solitary fibrous tumors. Ann Surg Oncol. 2017;24(13):3865–3871.
- Ghia AJ, Allen PK, Mahajan A, et al. Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery. 2013;72(2):203–209.
- Lee EJ, Kim JH, Park ES, Khang SK, et al. The impact of postoperative radiation therapy on patterns of failure and survival improvement in patients with intracranial hemangiopericytoma. J Neurooncol. 2016;127(1):181–190.
- Stessin AM, Sison C, Nieto J, et al. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int J Radiat Oncol Biol Phys. 2013;85(3):784–790.
- Kinslow CJ, Bruce SS, Rae AI, et al. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol. 2018;138(1):173–182.
- Rana N, Kim E, Jaboin J, et al. The role of adjuvant radiation in the management of solitary fibrous tumors of the central nervous system: a national cancer database analysis of 155 patients. Cureus. 2018;10(5):e2656.
- Champeaux C, Rousseau P, Devaux B, et al. A. Solitary fibrous tumours and haemangiopericytoma of the meninges. A retrospective study for outcome and prognostic factor assessment. Neurochirurgie. 2018;64(1):37–43.
- González-Vargas PM, Thenier-Villa JL, Sanromán Álvarez P, et al. Hemangiopericytoma/solitary fibrous tumor in the central nervous system. Experience with surgery and radiotherapy as a complementary treatment: a 10-year analysis of a heterogeneous series in a single tertiary center. Neurocirugia. 2020;31(1):14–23.
- Combs SE, Thilmann C, Debus J, et al. Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer. 2005;104(11):2457–2465.
- Okamoto N, Itokawa H, Moriya M, et al. Efficacy of preoperative radiation therapy in hyper-vascular solitary fibrous tumor. No Shinkei Geka. 2009;37(2):189–194.
- Cohen-Inbar O, Lee CC, Mousavi SH, et al. Stereotactic radiosurgery for intracranial hemangiopericytomas: a multicenter study. J Neurosurg. 2017;126(3):744–754.
- Copeland WR, Link MJ, Stafford SL, et al. Single-fraction stereotactic radiosurgery of meningeal hemangiopericytomas. J Neurooncol. 2014;120(1):95–102.
- Olson C, Yen CP, Schlesinger D, et al. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat Gamma Knife surgery. J Neurosurg. 2010;112(1):133–139.
- Veeravagu A, Jiang B, Patil CG, et al. CyberKnife stereotactic radiosurgery for recurrent, metastatic, and residual hemangiopericytomas. J Hematol Oncol. 2011;4:26.
- Kim JW, Kim DG, Chung HT, et al. Gamma Knife stereotactic radiosurgery for intracranial hemangiopericytomas. J Neurooncol. 2010;99(1):115–122.
- Reames DL, Mohila CA, Sheehan JP. Treatment of intracranial solitary fibrous tumors with gamma knife radiosurgery: report of two cases and review of literature. Neurosurgery. 2011;69(4):E1023–8.
- Ghose A, Guha G, Kundu R, et al. CNS hemangiopericytoma: a systematic review of 523 patients. Am J Clin Oncol. 2017;40(3):223–227.
- Trifiletti DM, Mehta GU, Grover S, et al. Clinical management and survival of patients with central nervous system hemangiopericytoma in the National Cancer Database. J Clin Neurosci. 2017;44:169–174.