Abstract
Objective
Human epididymis protein 4 (HE4) is a validated, complementary biomarker to cancer antigen 125 (CA125) for high grade serous ovarian carcinoma (HGSC). Currently, there are insufficient data on the utility of longitudinal HE4 measurement during HGSC treatment and follow up. We set to provide a comprehensive analysis on the kinetics and prognostic performance of HE4 with serial measurements during HGSC treatment and follow up.
Methods
This prospective study included 143 patients with advanced HGSC (ClinicalTrials.gov identifier: NCT01276574). Serum CA125 and HE4 were measured at baseline, before each cycle of chemotherapy and during follow up until first progression. Baseline biomarker values were compared to the tumor load assessed during surgery and to residual disease. Biomarker nadir values and concentrations at progression were correlated to survival.
Results
The baseline HE4 concentration distinguished patients with a high tumor load from patients with a low tumor load assessed during surgery (p<.0001). The baseline CA125 level was not associated with tumor load to a similar extent (p=.067). At progression, the HE4 level was an independent predictor of worse survival in the multivariate analysis (p=.002). All patients that were alive 3 years post-progression had a serum HE4 concentration below 199.20 pmol/l at the 1st recurrence.
Conclusion
HE4 is a feasible biomarker in the treatment monitoring and prognostic stratification of patients with HGSC. Specifically, the serum level of HE4 at first relapse was associated with the survival of patients and it may be a useful complementary tool in the selection of second line treatments. This is to the best of our knowledge the first time this finding has been reported.
Introduction
Epithelial ovarian cancer is an umbrella term that includes a heterogeneous group of malignancies, of which high grade serous ovarian carcinoma (HGSC) is the most common and aggressive [Citation1]. The survival rate of patients diagnosed with HGSC is poor (43% 5-year survival), mostly due to diagnosis at a late stage [Citation1]. The main pillar in primary HGSC treatment is the surgical removal of all visible tumor (complete cytoreduction) followed by platinum based chemotherapy [Citation2]. The implementation of precision drugs, specifically poly (ADP-ribose) polymerase (PARP)-inhibitors and bevacizumab, has been proven feasible in improving the survival of patients [Citation3,Citation4]. Although an adequate treatment response is generally obtained, most patients relapse and eventually develop a therapy-resistant end-stage disease [Citation5].
In the clinical setting, cancer antigen 125 (CA125) is currently the only biomarker widely used in the diagnosis and treatment monitoring of HGSC [Citation6]. The limitations of CA125 are well-known, and include insufficient detection of early stage disease, the presence of small amounts of tumor regardless of CA125 normalization, and the increase of serum CA125 in other cancers and benign conditions [Citation7]. Several studies have been conducted on the prognostic capacity of CA125 with conflicting results: there is evidence on the utility of preoperative serum CA125 level in predicting cytoreductibility [Citation8,Citation9], but contradictory results have also been reported [Citation10,Citation11]. The shortcomings of CA125 and the insidious nature of HGSC have sprouted comprehensive research on more satisfactory biomarkers in the diagnosis, treatment monitoring, and follow up of the disease [Citation12]. Human epididymis protein 4 (HE4) was first described by Kirchhoff et al. [Citation13], and it has since been proposed as a complement to CA125 in the diagnosis of HGSC [Citation14].
At present, there is promising, but insufficient evidence on the prognostic abilities of HE4 both in predicting optimal cytoreduction and cancer survival [Citation15–18]. However, there has also been conflicting results [Citation19], and currently there is no consensus on the clinical use of HE4 in the treatment monitoring and follow up of HGSC and the routine measurement of HE4 is not advocated to this end [Citation20]. Overall, there is a need for a comprehensive evaluation on the utility of HE4 throughout the treatment regimen of HGSC. In the current study, we evaluated the feasibility of HE4 in the treatment monitoring and prognostic stratification of HGSC by analyzing longitudinal serum HE4 concentrations.
Material and methods
Study population
This prospective study was conducted during 2009–2019 at the Turku University Hospital with the approval of the Ethics Committee in the Hospital District of Southwest Finland (ETMK 102 53/180/2009). The study was designed to evaluate biomarkers in the treatment monitoring of epithelial ovarian cancer (ClinicalTrials.gov identifier: NCT01276574) and 143 patients with histologically confirmed HGSC was included in the current cohort. Additional inclusion criteria were normal serum creatinine and/or glomerular filtration rate (GFR) [Citation21], at least three serum samples collected during primary therapy and a serologically and/or radiologically detected HGSC relapse [Citation22]. All participants signed a written consent at the time of enrollment and the study data were handled and analyzed according to the information security laws of the European Union. We followed the SPIRIT 2013 guideline when applicable (Supplementary data) [Citation23].
A team of gynecologic oncologists evaluated the up-front cytoreductibility and patients underwent a treatment scheme of either primary debulking surgery (PDS) with subsequent chemotherapy (N = 58) or if estimated inoperable in diagnostic laparoscopy, neoadjuvant chemotherapy (NACT) with interval debulking surgery (IDS) and adjuvant chemotherapy (N = 85) (). In the NACT group, 13 patients did not have debulking surgery due to progressive disease or general frailty. The operating gynecologic oncologist assessed the disease stage as stated by the International Federation of Gynecology and Obstetrics (FIGO) 2014 classification and a pathologist confirmed it from tissue biopsies. The operating team recorded the disease spread in the abdominal cavity and retroperitoneum to calculate a previously described disease dissemination score (range 0–21) (Table S1) [Citation24]. The disease dissemination score showed prognostic value in a previous study [Citation24]. Patients were divided into a low tumor load group (dissemination score 0–12) and a high tumor load group (dissemination score 13–21). In all patients treated with NACT, the disease dissemination score was calculated at diagnostic laparoscopy, before the initiation of chemotherapy. The operating team carefully assessed the amount of residual disease after cytoreductive surgery. The standard chemotherapy regimen included carboplatin and paclitaxel. Sixteen patients received single agent carboplatin because of general frailty. In 51 patients with advanced stage disease, bevacizumab was combined with the primary chemotherapy and continued as a maintenance therapy for 15 months or until first relapse (). In Finland, the first line bevacizumab was available for high risk patients from 2013 forward. After primary therapy, the patients’ response to primary therapy was evaluated by a multidisciplinary tumor board with radiological and serological criteria [Citation22]. Post-progression therapies included chemotherapy regimens indicated for the treatment of HGSC, i.e., pegylated liposomal doxorubicin (PLD), dose dense paclitaxel, and gemcitabine, alone or in combination with a platinum agent and bevacizumab.
Table 1. Patient characteristics.
Sample collection and analyses
Venous whole blood samples (10 ml) for the HE4 and CA125 analyses were drawn into serum separation tubes at baseline (before any oncological treatments), before each triweekly cycle of primary chemotherapy and during standard follow up visits at the outpatient clinic approximately every 3–6 months until first relapse. After incubation at room temperature, the samples were centrifuged, and the separated serum was aliquoted. We determined the serum CA125 concentrations with the clinically well-established ECLIA method on the Cobas e 601 instrument or manually with the EIA method (Fujirebio Diagnostics Inc., Malvern, PA, USA). In previous studies, these two methods have shown good correlation [Citation25]. Serum HE4 concentrations were similarly determined with the EIA method (Fujirebio Diagnostics Inc., Malvern, PA, USA).
Statistical analyses
The biomarker medians, 25th and 75th quartiles for each time point were calculated and the kinetics of the biomarkers during primary treatment were assessed. The biomarkers kinetics were first evaluated separately for the NACT and PDS groups with subsequent assessment of the combined treatment arms (NACT + PDS). We evaluated the distribution of the biomarker values at each time point and made a logarithmic transformation to correct for the non-Gaussian skewness of the data. The biomarker means of baseline serum samples were compared to different end points with the t-test or the one-way analysis of variance (ANOVA). The progression-free interval (PFI) was dichotomized to progression vs. no progression at a cut off time of 6 months to evaluate the potential of the biomarkers to detect platinum resistant progression. The nadir values for both HE4 and CA125 were calculated, as the nadir value of CA125 has been associated with the outcome of patients [Citation26,Citation27], and it was defined as the lowest serum biomarker value during primary treatment or within three months after the conclusion of treatments. The survival of different subgroups was evaluated in a time-to-event fashion with Kaplan–Meier’s estimator curves and the statistical significance was evaluated with the log rank test and/or Cox regression analysis. A stepwise selection process was used in the multivariate survival analysis and the biomarker medians were used as cut off values for the division of patients. Statistical analyses were done with the IBM SPSS software (IBM Corp., Released 2019, IBM SPSS Statistics for Macintosh, Version 26.0, IBM Corp., Armonk, NY, USA). p<.05 was considered significant.
Results
The serum baseline HE4 concentration correlates with tumor burden
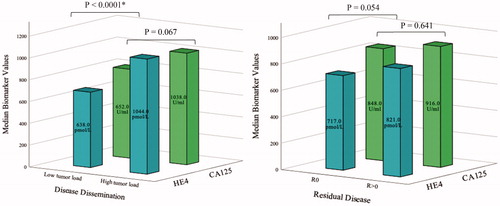
The longitudinal kinetics of serum CA125 and HE4 were similar during primary treatment in both treatment arms (PDS and NACT) (Tables S2 and S3). The biomarker kinetics were assessed carefully as some patients lacked serum samples in an individual time point; however, we detected no discrepancies in the longitudinal biomarker profiles. The median baseline concentration of CA125 was marginally higher in the PDS group compared to NACT (918.00 U/ml and 838.68 U/ml); however, the difference was not statistically significant (p=.998). Similarly, the median baseline HE4 concentrations were comparable in the treatment arms (PDS: 750.52 pmol/l and NACT: 755.52 pmol/l, p=.468). The treatment arms were combined for the evaluation of biomarker correlation to tumor burden. The baseline HE4 concentration was associated with the tumor burden stated during surgery (cytoreductive surgery in PDS patients and diagnostic laparoscopy in NACT patients) as the serum HE4 was significantly higher in patients with substantial tumor burden (dissemination score 13–21) than in patients with less extensive tumor growth (dissemination score 0–12) (p<.0001, ). The baseline CA125 was not to a similar extent associated with tumor burden (p=.067, ). Neither baseline CA125 nor HE4 were significantly associated with the amount of residual disease after cytoreductive surgery (p=.641 and p=.054, respectively). However, the baseline HE4 level was found to be trending toward statistical significance and it might be clinically relevant in evaluating the feasibility of up front debulking (R0 HE4 median 716.80 pmol/l, R > 0 mm HE4 median 829.02 pmol/l). We detected no association with baseline CA125 or HE4 to the PFI or OS.
The nadir CA125 and HE4 values predict platinum resistant disease
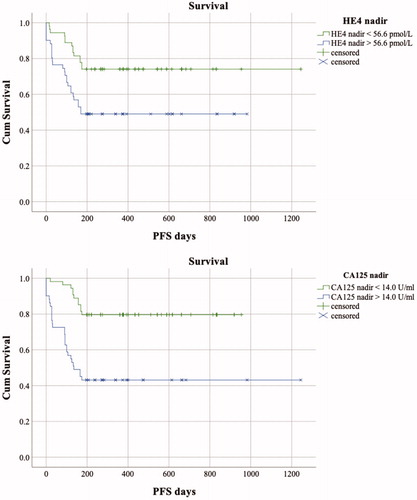
The median PFI of patients was 9.53 months (interquartile range 14.53 months). Patients receiving bevacizumab maintenance had a significantly longer PFI (median 14.20 months, IQR 17.93) than patients treated with conventional chemotherapy only (median 9.10 months, IQR 12.47) (p=.027, log rank test). Nadir values were evaluable for 71 patients of which 26 (36.62%) patients later developed rapid, platinum resistant relapse within 6 months after the conclusion of primary chemotherapy. The nadir value was reached simultaneously by the assays in 29 patients (40.85%). An earlier HE4 nadir was detected in 19 (26.76%) patients and 23 (32.39%) patients had a later HE4 nadir compared to the CA125 assay. The median nadir values were 14.00 U/ml (IQR 20.00) and 56.58 pmol/l (IQR 54.17) for the CA125 and HE4 assays, respectively. In the platinum resistant subgroup, the median CA125 and HE4 nadir values were 28.00 U/ml (IQR 41.00) and 87.68 pmol/l (IQR 103.33), respectively. In contrast, the median CA125 and HE4 nadirs in platinum sensitive subgroup were 10.50 U/ml (IQR 11.30) and 49.70 pmol/l (IQR 27.07), respectively. The nadir CA125 and HE4 levels were significantly elevated in patients who developed platinum resistant disease (p<.0001 both assays). The relationship of the nadir values to the PFI was further examined in a time-to-event fashion, and the Kaplan–Meier survival curves with the log rank test verified the above-mentioned results (CA125 p<.0001 and HE4 p=.008) ().
Figure 2. The Kaplan–Meier curves of progression free survival of patients with serum biomarker nadir values below and exceeding the nadir cut offs (medians). The HE4 and CA125 nadir values >56.58 pmol/l and >14.00 U/ml were significantly correlated with platinum resistant progression (log rank test, p=.008 and p<.0001, respectively).
The serum HE4 concentration at progression predicts the survival of patients
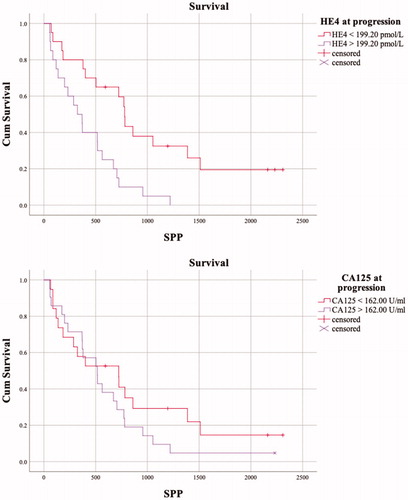
The follow up time of the study ranged from 1.5 months to 10.2 years, with a median follow up time of 2.5 years. The follow-up times were shortest (below 6 months) for four patients who developed fatal, rapidly progressing diseases during NACT. After disease relapse, the median survival of patients was 16.0 months (survival post-progression, SPP). At progression, the serum CA125 and HE4 concentrations showed median values of 162.00 U/ml and 199.20 pmol/l, respectively. Interestingly, elevated serum HE4 concentrations of >199.20 pmol/l at progression were significantly associated with a worse overall survival (univariate analysis p<.0001, ). No such correlation was detected with the CA125 assay (p=.13). In the univariate analysis, elderly patients (>65 years) and patients treated with NACT had worse overall survivals than younger patients and patients treated with PDS (p=.008 and p=.002, respectively, ); however, the findings were not confirmed in the multivariate analysis (p=.19 and p=.07, respectively, ). In the multivariate model, a HE4 serum concentration of >199.20 pmol/l was independently associated to worse OS (). Other covariates, such as disease stage, patients age, treatment strategy, residual disease or CA125 > 162.00 U/ml at progression were not independently associated to the OS of patients ().
Table 2. Hazard ratios with 95% confidence intervals (CIs) of OS by different clinical variables of HGSC patients (N = 40) using the log rank test and Cox’s proportional hazards model.
As OS has been suggested to be a suboptimal end point in cancer patients with a long SPP (>6 months) [Citation28], we set to further strengthen our results on the association of serum HE4 at progression and survival utilizing SPP. Still, a serum HE4 concentration of >199.20 pmol/l was significantly associated with a worse survival after the detection of progression in both the univariate (log rank, p=.001) and the multivariate model (p=.001) (). An identical multivariate model with the same covariates was used in the SPP and OS analyses and the median HE4 > 199.20 pmol/l at progression remained the only independent predictor of shorter SPP. Indeed, less than 50% of the patients with an HE4 concentration >199.20 pmol/l at progression were alive for a year post-progression (). In contrast, 50% of the patients in the HE4 < 199.20 pmol/l group were still alive two years after the detection of progression (). All patients that were alive 3 years post-progression had a serum HE4 concentration below 199.20 pmol/l at the detection progression. The serum CA125 concentration at progression was not significantly associated with SPP (p=.252) ().
Figure 3. The Kaplan–Meier curves of survival post-progression (SPP) of patients (N = 40) with serum biomarker values below and exceeding the cut offs (medians) at progression. A serum HE4 concentration of >199.20 pmol/l was significantly associated with worse survival (log rank test, p=.001). A higher serum CA125 concentration at progression was not significantly associated with survival (p=.252).
Discussion
In the current study, we present a longitudinal analysis on the feasibility of HE4 as a complement to CA125 in HGSC treatment monitoring and in the prognostic stratification of patients. These results provide valuable fortification to the previous studies on HE4 in HGSC patients. Our main findings were the linkage between baseline HE4 and tumor burden, and the prognostic potential of HE4 at the time of progression. This is to the best of our knowledge the first time these findings have been reported.
The landscape of HGSC medical treatment is rapidly changing due to the implementation of PARP inhibitors and immunotherapy combinations to patient care. These novel treatment options are specifically changing the notion of recurrent disease and patients might live longer with a slowly progressing or stable disease. Consequently, the prognostic stratification of relapsed HGSC patients is a topic of interest as it aids clinicians in choosing the most feasible second line treatment. In the current study, HE4 showed promise as a tempting auxiliary tool for CA125 in clinical trials, as high HE4 serum levels at the time of relapse were independently connected to survival.
In clinical trials, an objective, noninvasive assessment method of tumor burden is highly needed for the timely stratification of patients and in the monitoring of the treatment response. In the present study, the baseline serum level of HE4, unlike CA125, divided patients with high tumor burden from patients with a less extensive tumor growth. Previous studies have suggested the preoperative serum level of HE4 to be predictive of the amount of residual disease in patients with ovarian cancer [Citation29–31]. Our study did not offer further support to these findings, although strongly elevated serum HE4 levels were trending toward patients with macroscopic residual disease. The association reported in previous studies between baseline HE4 and the amount of residual tumor is indicative of a connection between serum HE4 and tumor burden; however, the anatomic location of the tumor growth is central in determining the feasibility of surgical removal and the amount of residual disease is not necessarily determined by tumor volume.
The majority of HGSC patients relapse despite a satisfactory response to primary therapy. The prognostic relevance of the CA125 nadir has attracted clinical interest and the connection between an elevated CA125 nadir and shorter PFS has been described in earlier studies [Citation32–34]. The prognostic utility of the HE4 nadir has been less extensively studied, but there is modest evidence on the prognostic disadvantage of a higher HE4 nadir [Citation31]. Our results are in line with these previous findings, as elevated CA125 and HE4 nadir values were significantly correlated with a shortened PFI, specifically preceding a platinum resistant relapse. Interestingly, the HE4 nadir was quite low in the current cohort (57.23 pmol/l) compared to the validated cut off of 150.00 pmol/l recommended by the assay manufacturer for postmenopausal patients.
The strengths of this study include the strict inclusion criteria, prospective design, and uniform treatment strategies. Also, the response and progression evaluation criteria were carefully assessed for each patient in a clinical trial setup. The number of patients in the progression analyses was quite low (N = 40) and the exploratory results obtained in the current study need further validation. The cohort consisted of patients with advanced stage HGSC, and the prognostic abilities of HE4 in early stage disease need to be evaluated in future studies. A limitation of the study is the use of two different immunoassays in the analysis of CA125, although the assays have shown to be highly comparable in previous research [Citation25]. Another limitation is the long study admission time and the use of various post-progression chemotherapy regimens. However, this is typical in the clinical setting and cannot be avoided either in clinical trials as several factors affect the choice of the most appropriate second line treatment, i.e., platinum sensitivity/resistance, toxicity profile, distribution of disease, and patient preference [Citation35]. The long admission time may have influenced the criteria for up-front cytoreduction and the quality of the surgery. However, the number of patients treated with NACT did not change during the study period and the primary chemotherapy regimen was quite homogeneous throughout the admission period.
The current study provides emerging evidence on the prognostic benefit of serum HE4 measurement in patients with HGSC. The measurement of serum HE4 appears to be a feasible complementary tool in the evaluation of up front cytoreductibility and the prognostic stratification of patients at disease progression. In addition, the measurement of serum HE4 could aid clinicians in a timely detection of platinum resistant disease and offer support for the decision of comprehensive genomic profiling to find individual targeted therapies. Importantly, HE4 performed better or at least as well as CA125 in all of the time points evaluated: at baseline, nadir and in 1st relapse. Future studies with heterogeneous cohorts, including patients receiving novel targeted therapies, for the assessment of differences in the longitudinal kinetics and prognostic abilities of conventional biomarkers are warranted.
Supplemental Material
Download MS Word (109.3 KB)Acknowledgements
We thank Fujirebio Diagnostics for providing the HE4 kits.
Disclosure statement
The authors do not have any conflicts of interest to disclose.
Additional information
Funding
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296.
- Benedet JL, Bender H, Jones H, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70(2):209–262.
- Hall M, Bertelli G, Li L, et al. Role of front-line bevacizumab in advanced ovarian cancer: the OSCAR study. Int J Gynecol Cancer. 2020;30(2):213–218.
- Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol. 2018;29(6):1366–1376.
- Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011;12(12):1169–1174.
- Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43–51.
- Bast RC, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(S3):274–281.
- Chi DS, Venkatraman ES, Masson V, et al. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol Oncol. 2000;77(2):227–231.
- Kang S, Kim TJ, Nam BH, et al. Preoperative serum CA-125 levels and risk of suboptimal cytoreduction in ovarian cancer: a meta-analysis. J Surg Oncol. 2010;101(1):13–17.
- Barlow TS, Przybylski M, Schilder JM, et al. The utility of presurgical CA125 to predict optimal tumor cytoreduction of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16(2):496–500.
- Mury D, Woelber L, Jung S, et al. Prognostic and predictive relevance of CA-125 at primary surgery of ovarian cancer. J Cancer Res Clin Oncol. 2011;137(7):1131–1137.
- Muinao T, Deka Boruah HP, Pal M. Diagnostic and prognostic biomarkers in ovarian cancer and the potential roles of cancer stem cells – an updated review. Exp Cell Res. 2018;362(1):1.
- Kirchhoff C, Habben I, Ivell R, et al. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45(2):350–357.
- Ferraro S, Panteghini M. Making new biomarkers a reality: the case of serum human epididymis protein 4. Clin Chem Lab Med. 2019;57(9):1284–1294.
- Trudel D, Têtu B, Grégoire J, et al. Human epididymis protein 4 (HE4) and ovarian cancer prognosis. Gynecol Oncol. 2012;127(3):511–515.
- Yuan C, Li R, Yan S, et al. Prognostic value of HE4 in patients with ovarian cancer. Clin Chem Lab Med. 2018;56(7):1026–1034.
- Kalapotharakos G, Asciutto C, Henic E, et al. High preoperative blood levels of HE4 predicts poor prognosis in patients with ovarian cancer. J Ovarian Res. 2012;5(1):20–29.
- Feng LY, Bin Liao S, Li L. Preoperative serum levels of HE4 and CA125 predict primary optimal cytoreduction in advanced epithelial ovarian cancer: a preliminary model study. J Ovarian Res. 2020;13(1):17.
- Ferraro S, Robbiano C, Tosca N, et al. Serum human epididymis protein 4 vs. carbohydrate antigen 125 in ovarian cancer follow-up. Clin Biochem. 2018;60:84–90.
- Colombo N, Sessa C, Du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;29(4):728–760.
- Kappelmayer J, Antal-Szalmás P, Nagy B. Human epididymis protein 4 (HE4) in laboratory medicine and an algorithm in renal disorders. Clin Chim Acta. 2015;438:35–42.
- John G, Rustin S, Vergote I, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011;21(2):419–423.
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207.
- Isoviita VM, Salminen L, Azar J, et al. Open source infrastructure for health care data integration and machine learning analyses. J Clin Oncol Clin Cancer Inform. 2019;3:1–16.
- Diamandis E, Fritsche H, Lilja H, et al. Tumor markers. Physiology, pathobiology, technology, and clinical applications. 1st ed. Washington (DC): AACC Press; 2003.
- Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol. 2005;16(1):47–50.
- Prat A, Parera M, Peralta S, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008;19(2):327–331.
- Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642–1649.
- Angioli R, Plotti F, Capriglione S, et al. Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol. 2013;128(3):579–583.
- Braicu EI, Fotopoulou C, Van Gorp T, et al. Preoperative HE4 expression in plasma predicts surgical outcome in primary ovarian cancer patients: results from the OVCAD study. Gynecol Oncol. 2013;128(2):245–251.
- Vallius T, Hynninen J, Auranen A, et al. Postoperative human epididymis protein 4 predicts primary therapy outcome in advanced epithelial ovarian cancer. Tumor Biol. 2017;39.
- Van Altena AM, Kolwijck E, Spanjer MJB, et al. CA125 nadir concentration is an independent predictor of tumor recurrence in patients with ovarian cancer: a population-based study. Gynecol Oncol. 2010;119(2):265–269.
- Xu X, Wang Y, Wang F, et al. Nadir CA-125 level as prognosis indicator of high-grade serous ovarian cancer. J Ovarian Res. 2013;6(1):31–38.
- Kang S, Seo SS, Park SY. Nadir CA-125 level is an independent prognostic factor in advanced epithelial ovarian cancer. J Surg Oncol. 2009;100(3):244–247.
- Ledermann JA, Kristeleit RS. Optimal treatment for relapsing ovarian cancer. Ann Oncol. 2010;21:vii218–vii222.