Abstract
Background
Penile cancer is an uncommon disease with poor prognosis when spread to more than one inguinal lymph node. Recommendations on chemo- and radiotherapy in treatment guidelines are based on low-grade evidence. There are to our knowledge no described population-based cohort with detailed information on given oncological treatment and survival data. The aim of this study is to investigate in detail how men with metastatic penile cancer have been treated with chemotherapy and radiotherapy over time, and how survival varies with N-stage and given treatment.
Material and methods
For this observational cohort study all men in Sweden diagnosed with penile cancer with lymph node- or distant metastases 2000-2015 were identified through the Swedish National Penile Cancer Register (NPECR). Medical records were retrieved and 325 men were confirmed to have metastatic penile cancer (Tany, c or pN1-3 and/or M1). Information on treatments was collected. Causes of death were retrieved from the National Cause of Death Register (CDR).
Results
Chemotherapy and/or radiotherapy were given to 172 (53%) of all men. The use of oncological treatments with curative intent increased significantly during the study period, from 30% of men with c/pN2-3 diagnosed 2000–2003 compared with 57% of men diagnosed 2012–2015. Ninety-three (29%) men received oncological treatments with curative intent of whom 85/93 (91%) had stage c/pN2-3M0. Survival decreased with higher N-stage, M1-stage, and absence of oncological treatment with curative intent. For men with c/pN3-stage, the engagement of pelvic lymph nodes was entailed with lower survival than pN3 based on extra-nodal extension (ENE).
Conclusion
The use of oncological treatment was below recommendations in guidelines but increased during the study period. Treatment was given predominantly to men with c/pN2-3 and M1-disease. Survival was higher among men treated with curative intent; this could be due to patient selection bias.
Introduction
Squamous cell carcinoma (SCC) of the penis is an uncommon disease. An age-standardized incidence of approximately one per 100.000 men has been reported from the USA and several European countries [Citation1–3]. In parts of Africa and South America, it is more common, with the highest incidence of 6 per 100,000 reported in Brazil [Citation4,Citation5]. In Sweden incidence has been approximately 2 per 100,000 men during the past 20 years [Citation6]. Since 2000, virtually all Swedish men with penile cancer are registered in the National Penile Cancer Register (NPECR).
The strongest prognostic factor is the stage, based on the TNM system [Citation7]. A previous study based on NPECR reported a 5-year relative survival of 82% for all patients, 94% for men with pN0, and 46% for men with pN1-3 [Citation6]. In men with pN3 disease, a five-year PeCSS (penile cancer-specific survival) of 33% has been reported [Citation8]. Men with pN3 due to ENE (extra-nodal extension) had a PeCSS of 42% at five years whereas for men with pN3 due to engagement of pelvic nodes survival dropped to 21% [Citation9].
Evidence of the efficacy of oncological treatment to men with penile cancer is limited. Results from small non-randomized studies indicate that it may be possible to improve the prognosis with the use of chemotherapy and radiotherapy [Citation10–13]. However, a systematic review in 2018 [Citation14] found insufficient evidence for recommending adjuvant radiotherapy. In addition, a recent retrospective study of 201 patients [Citation15] found that the indication for perioperative oncological treatment is still unclear. Most studies on the patterns of care of penile cancer have focused on surgery, however, a few studies have reported patterns of oncological treatments [Citation16–19].
Chemo- and radiotherapy constitute the standard of care for patients with SCC in the vulva, the cervix uteri, the head and neck, and the anus [Citation20–23]. All these tumors have obvious similarities to penile cancer.
Since 2000 EAU (European Association of Urology) Guidelines recommend neoadjuvant/adjuvant cisplatin-based chemotherapy to men with more than one lymph node metastasis [Citation24]. NCCN (National Comprehensive Cancer Network) recommends both chemotherapy and radiotherapy to men with the pN2-3 disease since 2013 [Citation25].
The Swedish National guidelines were first published in 2013, recommending adjuvant cisplatin-based chemotherapy to men with pN2-3. For men with fixed lymph nodes or relapse to regional lymph nodes, chemotherapy before surgery was recommended. Since 2015 neoadjuvant chemotherapy with PIC (paclitaxel, ifosfamide, and cisplatin) is recommended to men with pN2-3 disease [Citation26]. To men judged to be unfit for chemotherapy, and to those with a high risk of loco-regional recurrence, adjuvant radiotherapy with or without concomitant cisplatin can be considered.
Since 2013, a weekly national tumor board is held in Sweden. All men with newly diagnosed or recurrent penile cancer are discussed. While penile cancer surgery in Sweden is centralized to two hospitals since January 2015, oncological treatment remains decentralized.
The aims of the present study were twofold. First, to describe patterns of chemo and radiotherapy use in men with c/pN ≥1 or M1 penile cancer. Second, to examine outcomes in relation to tumor stage and treatment in a population-based cohort with complete follow-up.
Material and methods
Study design
We used data from three population-based registers: the NPECR, the Swedish Cancer Register (SCR) and the Cause of Death Register (CDR). NPECR has 99% completeness when compared to the SCR to which reporting is mandated by law [Citation6]. The under-reporting to the SCR for solid tumors has been estimated to 4% [Citation27]. The proportion of deaths with missing information in the CDR has been estimated to be around 1% [Citation28].
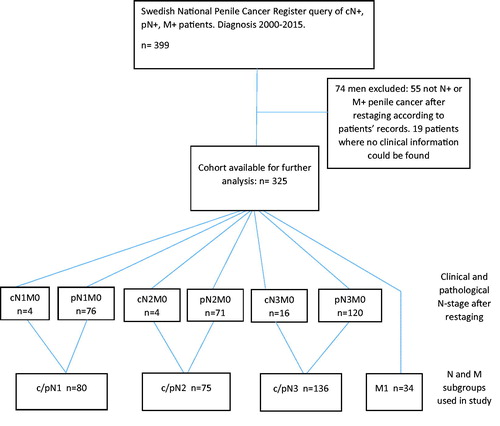
A total of 2139 men were identified in NPECR as diagnosed with penile cancer between 1 January 2000 and 31 December 2015. Of them, 399 met one of the following criteria: cN ≥ 1, pN ≥ 1, or M1. N- and M-stage as reported to NPECR were used only in this phase of the study. See for a flowchart of patient selection.
Medical records were retrieved and a re-staging of all patients N-and M-stages, according to TNM 2009 UICC 7th Edition [Citation7], was conducted based on information from medical records such as pathology- and imaging reports as well as results from physical examinations. Seventy-four men were excluded. The reasons were that metastases could not be verified (n = 53), lack of clinical information (n = 19), and other malignancy than penile cancer (n = 2). Of the 53 men without metastases, 31 were cN ≥ 1 in NPECR but converted to pN0 after re-staging. The final study cohort consisted of 325 men with TNM-stage confirmed to be cN ≥ 1, pN ≥ 1, and/or M1 penile cancer.
N-stage indicates the extent of tumor spread to lymph nodes, cN is clinical-stage, pN is the pathological stage, based on tissue samples. According to the TNM 2009 UICC 7th Edition, cN1 is one unilateral inguinal lymph node, cN2: multiple unilateral or bilateral inguinal lymph nodes, cN3: fixed inguinal nodal mass or pelvic lymphadenopathy, pN1: metastasis in one inguinal lymph node, pN2: metastasis in multiple or bilateral inguinal lymph nodes and pN3 metastasis in the pelvic lymph node, or extranodal extension of any metastasis [Citation7].
Of men, without distant metastases, 92% could be assigned a pN-stage based on information retrieved from medical records, the remaining 8% (n = 24), were assigned a cN-stage. Of the 24 men with cN ≥1 17 died from penile cancer. This supports the notion that a majority of these men really had lymph node metastases at diagnosis. Although a morphologically confirmed diagnosis is mandatory for decisions on oncological treatment, we chose not to exclude patients with clinically established N ≥ 1 since this would lead to loss of information from this population-based cohort. Cases with a clinically-based N-stage were merged with the corresponding pN-stage patients to form three groups: c/pN1 (pN1M0 + cN1M0), c/pN2 (pN2M0 + cN2M0), and c/pN3(pN3M0 + cN3M0). Men with distant metastases at diagnosis formed a separate group, M1. These groups were hereinafter used in all analyses. Clinical T-stage as registered in NPECR was used instead of pT-stage because the latter had more missing data. Ten men were registered as having noninvasive primary tumors, cT0,cTa, and cTis, however, all 10 had confirmed lymph node metastases, thereby meeting inclusion criteria.
Information on surgery is available in NPECR and has been described before [Citation6]. In this study, information from lymph node surgery was collected, since this is the most relevant surgery in relation to oncological treatment. Tumor involved margins are seldom a clinical problem in the management of lymph-node metastases in penile cancer. It is not registered in the NPECR and therefore it was not available for our study.
A database with the clinical information retrieved from the medical records was constructed, including the following parameters: cT-stage, c/pN-stage (for c/pN3 engagement of pelvic nodes and ENE were registered), M-stage, chemotherapy (type of regimen and intention of treatment), radiotherapy (dose, number of fractions, targets and intention of treatment), chemoradiotherapy (CRT) (dose, number of fractions, targets, type of chemotherapy and intention of treatment), reasons not to recommend oncological therapy and surgery to inguinal and pelvic lymph nodes (yes/no). Causes of death were obtained from the CDR in October 2018, 33 months after the last included man was diagnosed with penile cancer. Data on causes of death were linked to the database.
Modern medical terminology used for describing oncological treatments with chemo and radiotherapy before or after surgery varies. We have chosen to use the expression neoadjuvant for treatments given before surgery when clinically overt distant metastases have been excluded and treatment intent is curative. This is in line with EAU, NCCN, and Swedish guidelines. Accordingly, adjuvant treatment denotes treatments administered after radical surgery in a curative situation.
To enable comparisons over time, we split the cohort into four arbitrary time periods of equal duration: 2000–2003, 2004–2007, 2008–2011, and 2012–2015.
Statistics
Net survival was estimated by calculating the PeCSS using the Kaplan–Meier estimator. Patients were followed from diagnosis to death, emigration, or end of follow-up (October 2018), whichever occurred first. The event of interest was death from penile cancer and individuals who died from other causes were censored at their time of death. Hence, the PeCSS illustrates the probability of survival in a hypothetical world where penile cancer is the only possible cause of death. The analyses were stratified by c/pN1 vs. c/pN2, c/pN3, and M1; c/pN3 based on pelvic lymph node metastases vs. ENE; c/pN2 and c/pN3 in the curative situation, receiving oncological treatment vs. not receiving oncological treatment. Differences between groups were tested with the log-rank test. Cox proportional hazard model was employed to estimate hazard ratios (HRs) for penile cancer-specific mortality with 95% confidence intervals (CIs). Time since penile cancer diagnosis was used as the underlying time scale and the multivariable model was mutually adjusted for the stage, year of diagnosis, and age at diagnosis. The proportional hazards assumption was verified by studying the scaled Schoenfeld residuals. Trend analysis of the use of curative treatment over time periods of patients with c/pN2-3 was performed with a chi-squared test for trends in proportions.
R versions 3.5.3 were used for statistical analyses except for the unadjusted Cox regression and the trend analysis where version 3.6.1 was used.
p < 0.05 was considered statistically significant and all tests were two-sided.
The study was approved by the local Ethics Committee in Uppsala (Dnr 2015/520).
Results
Patient population and treatments
Patient characteristics and treatments are presented in . In total 172 (53%) men received chemo or radiotherapy at any time for any indication related to penile cancer. The remaining 153 (47%) men did not receive any oncological treatment. Reasons to abstain from oncological therapy were not specified for 83 of these 153 patients. For the remaining 70 the most common reason for men with c/pN1 was that treatment was not indicated (10 of 14) and for men with c/pN2-N3 that treatment was not indicated (19 of 48) or because of comorbidity or high age (17 of 48). For men with M1 who did not receive oncological treatment the most common reason stated was comorbidity or high age (6 of 8).
Table 1. Patient, disease, and treatment characteristics of 325 men with N + or M + penile cancer.
Ninety-three of the 172 treated patients received treatment with curative intent at some point, and of these 28 men later received palliative oncological treatment. In situations with curative intent, chemotherapy was used as a neoadjuvant and/or adjuvant treatment, while radiotherapy predominantly was adjuvant. In men with c/pN2-3M0 disease, 40% received chemo or radiotherapy with curative intent. Seventy-nine (24%) men were treated only in a palliative setting.
Adjuvant radiotherapy was given to 45 men. Of these, 31 were treated with fractions of 1.5-2 Gray to a total dose of at least 50 Gray. The penile base and inguinal and/or pelvic lymph nodes were radiotherapy targets. Palliative radiotherapy was given to 76 men. The most common targets were locoregional and bone metastases.
The proportions of patients receiving oncological treatment increased by higher N-stage. Cisplatin-based regimens were the most common for all indications and CRT was solely given to men with c/pN2-3 or M1, .
Table 2. Treatment with chemotherapy and radiotherapy, per setting and per N- and M-stage.
The proportion of men with c/pN2-3 receiving oncological therapy with curative intent increased during the most recent time period. During the first three periods; (2000–2003), (2004–2007), (2008–2011), 30%, 41% and 30% were treated compared with 57% among those diagnosed 2012–2015 (Supplementary material, Table 3a; Table 3b). There was a statistically significant trend toward increased use of curative treatments for men with c/pN2-3 when comparing the four time periods.
Surgery with curative intent to inguinal and pelvic lymph nodes was performed in 81 and 49% of all men, respectively. All men who underwent pelvic surgery also had inguinal surgery. Of men treated with oncological therapy with curative intent, 94% had lymph node surgery, while among men not treated in this situation 76% had lymph node surgery. During follow-up, 220 men died. In 163 (74%) men, penile cancer was recorded as the primary cause of death.
Survival
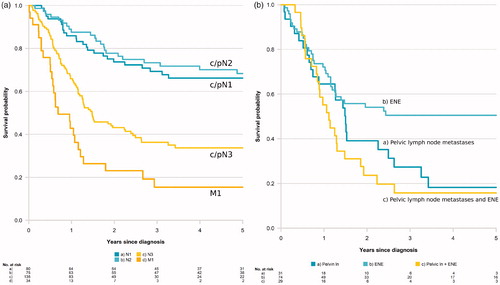
PeCSS varied with the TNM stage, a poorer survival was observed in men with higher compared with lower N-stage and in M1 disease compared with M0 disease. The median overall survival among the 34 men in the M1-group was 9.0 months, with an interquartile range of 3.8–15 months. In addition, the c/pN3 subgroup based on metastases to pelvic lymph nodes had a worse survival than men with c/pN3 based on ENE ().
Figure 2. Kaplan–Meier curves for penile cancer specific survival by (a) c/pN- and M-stage (b) c/pN3 subgroups (pelvic lymph node metastases and/or ENE (extranodular extension).
PeCSS of patients with c/pN2-3 disease per treatment group is presented in , showing prolonged survival among men with c/pN2-3 disease in a situation with curative potential receiving oncological treatment compared to men with the same stage and situation not receiving oncological treatment.
Figure 3. Kaplan–Meier curves for penile cancer specific survival by c/pN2 and c/pN3 stage and by patients in curative setting receiving oncological treatment or not. (a) c/pN2 receiving oncological treatment with curative intent, (b) c/pN2 not receiving oncological treatment with curative intent, (c) c/pN3 receiving oncological treatment with curative intent, (d) c/pN3 not receiving oncological treatment with curative intent. Median age per group in curves.
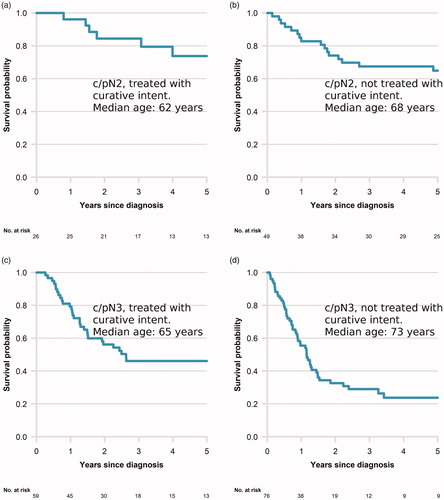
The median age in treatment groups varied as shown in the figure with lower age in treated groups. We abstained from further statistical testing due to low numbers.
Unadjusted Cox regression analysis including stage, time period, and age resulted in statistically significant hazard ratios for: c/pN3 and M1 versus c/pN1 with a HR of 2.9 [95% CI: 1.8–4.4] and 5.6 [95% CI: 3.2–9.7] (Supplementary material Table 4). The multivariable Cox analysis gave similar results: c/pN3 and M1 being the only factors that resulted in a HR that reached statistical significance, the HR estimates were in the same size order as in the unadjusted analysis (Supplementary material Figure 4).
Discussion
In the present study, we report on patterns of oncological treatment of Swedish men diagnosed with penile cancer with metastases to regional lymph nodes or other organs from 2000 to 2015. To our knowledge, this is the first study with detailed information on actual given oncological treatment to a population-based cohort of penile cancer patients. Previous studies have either presented larger materials but without information on the intent of treatment or details on chemo- and radiotherapy, or smaller series of patients but with detailed information.
Compared to results from earlier studies we found a slightly higher median age, likely reflecting the population-based setting [Citation9,Citation15]. In our series, almost half of the patients with N-positive disease were staged as c/pN3, which is higher compared to other reports based on the 7th edition of the UICC TNM-classification. The proportion of men with lymph-node metastases staged as c/pN3 has varied between 19% in the UK [Citation8], 30% in the US NCDB (National Cancer Database) [Citation18], and 41% in a cohort from the Memorial Sloan–Kettering Cancer Centre [Citation19]. These differences may reflect the selection of patients, but also the intensity of nodal staging procedures.
The use of chemo and radiotherapy during the period under study was low but increasing, overall 40% of men with c/pN2-N3 received treatment with curative intent. Although recommendations on oncological therapy have varied, chemotherapy as part of curative treatment to men with pN2-3 has been recommended in international and national guidelines during the entire study period. During the most recent time period 57% of men with c/pN2-3 received oncological therapy with a curative intent which is comparable to results in other studies, for example, the NCDB-report where 53% of men with c/pN3 and 40% of men with c/pN2 received chemotherapy [Citation18].
The increase of oncological treatments corresponds in time with efforts to improve penile cancer care in Sweden, for example, the preparation and distribution of updated national guidelines, centralization of curative surgery, and the advent of national multidisciplinary tumor boards. The increased use of oncological treatments could possibly be explained as an effect of these improvements.
Although treatment intensity increased during the study period, survival for the entire group did not improve. As this is a retrospective study and no information was available on comorbidity or socioeconomic factors, unconfounded comparisons cannot be made. The absence of increased survival should not be interpreted as an indicator of lack of treatment efficacy but could be explained by differences in comorbidity or socioeconomic status between groups.
Survival was better among men receiving oncological treatment with curative intent compared to men with the same stage and curative potential not receiving oncological treatment. This finding is possibly biased due to confounding by indication since men receiving treatment were younger and also probably were selected due to a low grade of comorbidity. On the other hand, when the proportion of men receiving treatment increases, the burden of comorbidity will probably also increase, which in turn could affect the survival in the treated group negatively. Survival can also be affected by immortal time bias. Patients can end up without treatment due to rapidly progressive disease which can lead to an apparent survival advantage for the treatment group.
The present findings confirm the relatively good prognosis in men with c/pN1 and the poor prognosis in men with c/pN3 and M1 disease. We also show that survival differs between c/pN3 subgroups with pelvic lymph node metastases being associated with a poorer prognosis than ENE, which supports results from previous studies [Citation29].
In the present study, we used the TNM 2009 UICC 7th edition [Citation7]. A new version of the TNM staging system was published in 2017 and changed the definitions of pN1 and pN2-stages. In the 2017 edition, pN1 is defined as 1–2 unilateral lymph node metastases compared with 1 metastatic lymph node in the older version. If staging would have been based on the new version, the c/pN2-group would have become smaller and the prognosis of the group would have been affected negatively.
The strengths of our study include the population-based setting within a health care system aiming to provide equal care to all residents. In addition, the follow-up of patients was virtually complete. Moreover, using medical records to access information on the intent of treatment and given therapy made it possible to more accurately define treatment groups and avoid simplified subgrouping, for example, chemotherapy yes or no. This enabled us to report actual given treatment also in the palliative setting which has not been done before.
Limitations of the study include the retrospective design and the lack of information on comorbidity and socioeconomic status. Although this is a large study of men with penile cancer, the disease is still very rare. Therefore, our detailed presentation of treatment data (e.g., Supplemantary material Table 3a; Table 3b) includes few patients in each treatment group, preventing valid comparisons between subgroups. Another limitation is the long study period, during which diagnostic procedures have evolved. The use of computed tomography and fluorodeoxyglucose-PET has increased during the study period and this has influenced staging and possibly caused stage migration which makes conclusions on different stages difficult.
Analysis for HPV status, an important prognostic and predictive factor for other types of squamous cell carcinomas was not available since it was not routinely used during the study period. Moreover, with a study cohort based on register data, there is a risk that wrongly classified cases, that is, men with false-negative nodal status, are excluded.
Retrospective analyses of data from population-based registers are nevertheless of great importance for epidemiological studies, hypothesis generation, and evaluation of adherence to guidelines. The present study, which was made possible by NPECR, supports the notion that the introduction of national guidelines can contribute to a higher treatment intensity for men with high-risk penile cancer. But when it comes to comparing new therapies, with those presently used, Randomized Clinical Trials (RCT) are the golden standard. For that reason, results from the International Penile Advanced Cancer Trial (InPACT) are much awaited [Citation30].
In summary, we describe patterns of actually given chemo- and radiotherapy, and PeCSS, in a population-based cohort of men with penile cancer with metastases to lymph nodes or other organs.
Supplemental Material
Download MS Word (19.9 KB)Supplemental Material
Download PNG Image (201 KB)Supplemental Material
Download MS Word (12.7 KB)Supplemental Material
Download MS Word (18.7 KB)Acknowledgments
This project was made possible by the continuous work of the National Penile Cancer Register of Sweden steering group. The Regional Cancer Centre of the Uppsala Örebro Health Care Region contributed to data base management and statistical analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are in parts from NPECR which welcomes research based on the register. Data in the study data base collected from medical records can only be made available after ethical approval.
Additional information
Funding
References
- Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
- Global Cancer Observatory [Internet]. Lyon (France): GCO; [cited 2019 November 18]. Available from: https://gco.iarc.fr/
- Coelho RWP, Pinho JD, Moreno JS, et al. Penile cancer in Maranhão, Northeast Brazil: the highest incidence globally? BMC Urol. 2018;18(1):50.
- Christodoulidou M, Sahdev V, Houssein S, et al. Epidemiology of penile cancer. Curr Probl Cancer. 2015;39(3):126–136.
- Kirrander P, Sherif A, Friedrich B, et al. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int. 2016;117(2):287–292.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours [Internet]. Hoboken (NJ): Wiley; 2011 [cited 2019 November 18]. Available from: https://www.wiley.com/en-us/TNM+Classification+of+Malignant+Tumours%2C+7th+Edition-p-9781444358964
- Veeratterapillay R, Teo L, Asterling S, et al. Oncologic outcomes of penile cancer treatment at a UK supraregional center. Urology. 2015;85(5):1097–1103.
- Graafland NM, van Boven HH, van Werkhoven E, el al. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol. 2010;184(4):1347–1353.
- Tang DH, Djajadiningrat R, Diorio G, et al. Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol Oncol. 2017;35(10):605.e17–605.e23.
- Sharma P, Djajadiningrat R, Zargar-Shoshtari K, et al. Adjuvant chemotherapy is associated with improved overall survival in pelvic node–positive penile cancer after lymph node dissection: a multi-institutional study. Urol Oncol Semin Orig Investig. 2015;33(11):496.e17–496.e23.
- Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Cin Oncol. 2010;28(24):3851–3857.
- Franks KN, Kancherla K, Sethugavalar B, et al. Radiotherapy for node positive penile cancer: experience of the Leeds Teaching Hospitals. J Urol. 2011;186(2):524–529.
- Robinson R, Marconi L, MacPepple E, et al. Risks and benefits of adjuvant radiotherapy after inguinal lymphadenectomy in node-positive penile cancer: a systematic review by the European Association of Urology Penile Cancer Guidelines Panel. Eur Urol. 2018;74(1):76–83.
- Necchi A, Pond GR, Raggi D, et al. Clinical outcomes of perioperative chemotherapy in patients with locally advanced penile squamous-cell carcinoma: results of a multicenter analysis. Clin Genitourin Cancer. 2017;15(5):548.e3–555.e3.
- Burt LM, Shrieve DC, Tward JD. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 2014;88(1):94–100.
- Chipollini J, Chaing S, Peyton CC, et al. National Trends and Predictors of Locally Advanced Penile Cancer in the United States 1998-2012. Clin Genitourin Cancer [Internet]. 2017 [cited 2017 Nov 30]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S1558767317302410.
- Joshi SS, Handorf E, Strauss D, et al. Treatment trends and outcomes for patients with lymph node–positive cancer of the penis. JAMA Oncol. 2018;4(5):643.
- Moses KA, Winer A, Sfakianos JP, et al. Contemporary management of penile cancer: greater than 15 year MSKCC experience. Can J Urol. 2014;21(2):7201–7206.
- Weinberg D, Gomez-Martinez RA. Vulvar cancer. Obstet Gynecol Clin North Am. 2019;46(1):125–135.
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) [Internet]. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane database Syst Rev. 2010. Jan 20 [cited 2019 May 31];(1):CD008285. Available from: http://doi.wiley.com/10.1002/14651858.CD008285
- Pignon J-P, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14.
- Spithoff K, Cummings B, Jonker D, et al. Chemoradiotherapy for squamous cell cancer of the anal canal: a systematic review. Clin Oncol. 2014;26(8):473–487.
- EAU Guidelines: Penile Cancer | Uroweb [Internet]. [cited 2019 Nov 18]. Available from: https://uroweb.org/guideline/penile-cancer/
- NCCN Clinical Practice Guidelines in Oncology Penile Cancer [Internet]. [cited 2019 Nov 18]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf
- Nationellt vårdprogram peniscancer [Internet]. [cited 2019 Nov 18]. Available from: https://www.cancercentrum.se/samverkan/cancerdiagnoser/penis/vardprogram/
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33.
- Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Li Z, Guo S, Wu Z, et al. Subclassification of pN3 staging systems for penile cancer: proposal for modification of the current TNM classification. Urol Oncol Semin Orig Investig. 2017;35(9):543.e1–543.e6.
- Crook J. Radiotherapy approaches for locally advanced penile cancer. Curr Opin Urol. 2017;27(1):62–67.