 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and purpose
Proton therapy has been proposed as a technique to improve the long-term quality of life of breast cancer patients. This is due to its ability to reduce the dose to healthy tissue compared to conventional X-ray therapy. The aim of this study was to investigate the risk of secondary carcinogenesis due to proton therapy compared to hybrid IMRT for breast treatments.
Material and methods
In this study, the Pinnacle treatment planning system was used to simulate treatment plans for 15 female left-sided whole breast cancer patients with deep inspiration breath hold scans. Two treatment plans were generated for each patient: hybrid intensity modulated radiotherapy (h-IMRT) and intensity modulated proton therapy (IMPT). Using the dose-volume histograms (DVHs) from these plans, the mean lifetime attributed risk (LAR) for both lungs and the contralateral breast were evaluated using the BEIR VII and Schneider full risk models.
Results
The results from both risk models show lower LAR estimates for the IMPT treatment plan compared to the h-IMRT treatment plan. This result was observed for all organs of interest and was consistent amongst the two separate risk models. For both treatment plans, the organs from most to least at risk were: ipsilateral lung, contralateral breast, and contralateral lung. In all cases, the risk estimated via the BEIR VII model was higher that the Schneider full risk model.
Conclusion
The use of proton therapy for breast treatments leads to reduced risk estimates for secondary carcinogenesis. Therefore, proton therapy shows promise in improving the long term treatment outcome of breast patients.
Introduction
Breast cancer is the most commonly diagnosed cancer in Australian women [Citation1]. A common way of treating this disease is via external beam radiation therapy, where high energy X-rays are used to deliver high doses of radiation to the tumour site. These procedures however, come at the risk of irradiating and damaging nearby healthy tissue. If these damaged cells are repaired incorrectly it can result in the formation of second primary cancers (SPCs) at some later point in the patient’s life [Citation2]. This biological phenomenon is known as radiation carcinogenesis [Citation3].
For breast cancer patients, there have been numerous clinical reports showing an increased risk of non-breast SPC after their radiotherapy treatment [Citation4,Citation5]. Second lung cancer and second sarcoma being the most common SPC found. However, over the last decade, there has been an increasing number of new radiotherapy techniques, such as intensity modulated radiotherapy (IMRT), intensity-modulated arc therapy (IMAT), intraoperative radiotherapy (IORT), and proton beam therapies. It is important to continue to estimate the effects that these different dose distributions have on the risk of SPC after radiotherapy.
Several studies have already been performed that estimate the risk of developing SPC due to breast radiotherapy. Donovan et al. [Citation6] investigated the organ specific cancer incidence risks for early breast cancer patients using the risk models presented by the BEIR VII committee. This study investigated the lifetime attributed risks (LARs) for various treatment techniques including: whole breast radiotherapy (WBRT), accelerated partial beast irradiation (APBI), and various simultaneous integrated boost (SIB) treatments. The study showed that the SIB treatments displayed a higher risk of SPC induction in the lungs, compared to the WBRT and APBI treatments. Furthermore, the LAR estimates were shown to decrease with age of exposure, implying that younger patients are more likely to develop SPC.
Another study, conducted by Santos et al. [Citation7], investigated the LAR for several radiation therapy treatments for breast cancer, including: WBRT, APBI, segmented breast treatment, and MammoSite boost treatment. For all treatment techniques, the sites which corresponded to the greatest LAR estimates were the lungs and the contralateral breast. It was also found that the LAR estimates for the segmented breast and MammoSite boost treatments were lower than those of the WBRT and APBI treatments.
Furthermore, Johansen et al. [Citation8] studied the risk of SPC induction for the contralateral breast using both linear and non-linear risk models. The treatments investigated in this study included: conventional radiotherapy, IMRT, and IMAT. The results showed an increase in risk for IMRT techniques compared to the conventional radiotherapy and IMAT treatments. Risk estimates using the non-linear risk model were also observed to be lower compared those of the linear model for all three treatment techniques.
For all the treatment techniques mentioned in the studies above [Citation6–8], high energy photons (X-rays) are used to deliver high doses of radiation to the tumour sites. In theory, it may be advantageous to use protons instead of X-rays as the source of radiation. This is due to the proton Bragg peak, which greatly reduces the entry and exit radiation dose, thus sparing the healthy tissue surrounding the tumour. A recent review by Corbin and Mutter proposes that proton beam therapy represents a promising approach to improve long-term treatment outcomes [Citation9].
A study by De Rose et al. [Citation10] investigated the role of intensity modulated proton therapy (IMPT) in the regional node irradiation of breast cancer compared to IMAT. The results showed a significant decrease in the excess absolute risk estimates for the contralateral breast and both lungs. A more recent study by Paganetti et al. [Citation11] also compared the risk of SPC induction for proton beam therapy compared to IMAT and conventional X-ray radiotherapy for breast patients receiving comprehensive nodal irradiation. It was found that IMAT techniques increased the risk compared to both conventional X-ray radiotherapy and proton beam therapy.
In this study, the LAR of SPC induction is investigated for two treatment techniques: hybrid intensity modulated radiotherapy (h-IMRT) and IMPT. The organs of interest are both the ipsilateral and contralateral lung and the contralateral breast.
Material and methods
Treatment planning
The Pinnacle3 treatment planning system (Philips Radiation Oncology Systems, Fitchburg, WI) was used to simulate treatment plans for 15 female left-sided whole breast cancer patients, scanned in deep inspiration breath hold (DIBH). Two treatment plans were created for each patient: h-IMRT and IMPT. H-IMRT plans utilised opposed tangential beams with energies of 6, 10, or 18 MV, where 70% of the dose was delivered by open fields and the remaining 30% was delivered by IMRT fields. The IMPT plans had a single en-face pencil beam scanning proton beam. One field was deemed sufficient to treat the volume, applying extra fields would unnecessarily increase both healthy tissue irradiation and treatment time [Citation12].
40.05 Gy in 15 fractions was prescribed for all plans. The primary evaluation goal for all plans was >98% coverage of the PTV with 95% of the prescribed dose, with maximum effective dose <107%. The organ at risk constraints was based on our department protocol and consists of a heart mean dose <3 Gy, V21.5 Gy <10%, and a left lung V18 Gy <15%.
Since there are 15 patients and two treatment techniques, a total of 30 treatment plans were generated for this study. For each treatment plan, the cumulative dose-volume histograms (DVHs) were exported for both lungs and the contralateral breast. To determine LAR estimates, the DVHs were imported into MATLAB (MathWorks, Natick, MA, USA) for analysis.
Secondary malignancy risk estimates
The risk of secondary malignancy was estimated with two separate risk models presented by the BEIR VII committee and Schneider et al. In the first model, the BEIR VII committee recommends EquationEquation (1)(1)
(1) for risk computation [Citation13]:
(1)
(1)
These functions are summed over the age of attainment from
to 90 years, where the age of exposure
and the latency period
were set to 30 years and 5 years, respectively. The weightings
and
as well as the baseline cancer risk
are presented in for each organ of interest.
represents the Australian female survival rate for a given age
These were obtained from the 2015–2017 life span tables from the Australian Bureau of Statistics [Citation14].
Table 1. Summary table for the site specific parameters used for the LAR estimate for the BEIR VII risk model [Citation13].
EquationEquation (1)(1)
(1) also contains the functions:
and
which are the excess relative risk and excess absolute risk, respectively. For the BEIR VII model [Citation13], these functions are described in EquationEquation (2)
(2)
(2) .
(2)
(2)
The parameter is defined in EquationEquation (3)
(3)
(3) .
(3)
(3)
The mean dose received by an organ was obtained from the DVHs and was used as an estimate for the total dose received by an organ Furthermore,
and
are site specific parameters based on the committee’s analysis of data for the solid cancer incidence of different organs [Citation13]. These values are listed in .
Table 2. Summary table for site specific parameters for ERR and EAR estimates using the BEIR VII risk model [Citation13].
For the Schneider et al. model, the LAR was similarly calculated using EquationEquation (1)(1)
(1) . To estimate the
Schneider et al. [Citation15] recommends the following equation:
(4)
(4)
In this equation, is the differential volume of the organ that was exposed to a dose
This was obtained the differential DVHs. Similar to the BEIR VII model,
is a modifying function containing population dependent variables such as the age of exposure
and age of attainment
(5)
(5)
Again, and
are site specific parameters obtained from the life span study cohort of Hiroshima and Nagasaki atomic bomb survivors, with values listed in [Citation16,Citation17]. EquationEquation (4)
(4)
(4) also includes a mechanistic model,
for carcinoma induction due to radiation dose (
), which accounts for cell death and fractionation effects [Citation18].
(6)
(6)
(7)
(7)
Table 3. Summary table for site specific parameters for ERR and EAR estimates using the Schneider et al. risk model [Citation16,Citation17].
The repair/repopulation parameter, and the proportionality constant
are listed in for the organs of interest. It was also assumed that
3 Gy for all organs. The prescribed dose to the target volume,
and dose per fraction,
for all treatment plans were 40.05 Gy and 2.67 Gy, respectively.
The ERR for the Schneider et al. model was calculated by multiplying the EAR by the term presented in .
Results
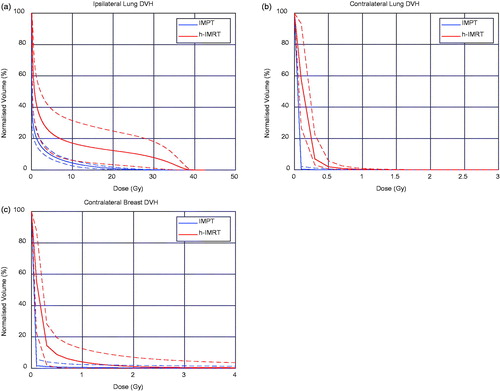
shows the volume normalised DVHs for the lungs and contralateral breast. It is evident that the IMPT treatment plans result in less dose to the lungs and contralateral breast then that of the h-IMRT DVH plot. It is also observed that there is less spread in the planned doses for the IMPT treatment plans compared to the h-IMRT plans.
Figure 1. The volume-normalised cumulative DVHs for (a) the ipsilateral lung, (b) the contralateral lung, and (c) the contralateral breast. The solid lines represent the mean DVH for all patients, while the dashed lines represent the upper and lower DVH curves.
shows some of the key dosimetric values for the lungs, contralateral breast, heart, and the left anterior descending (LAD) artery. The heart regions have been included since it is an important organ at risk for breast radiotherapy. It is shown that in all cases the IMPT treatment plans result in reduced dose to these organs at risk.
Table 4. Summary of the key mean dosimetric values with the corresponding ranges for the lungs, contralateral breast, heart, and the left anterior descending artery.
displays the risk estimates for the two treatment plans, which were calculated using the BEIR VII risk model. For the ERR and EAR estimates presented, an age of attainment of 70 years was applied. From this table, it is clear that the average estimates for ERR, EAR, and LAR are all lower for the IMPT technique. In particular, the ipsilateral lung LAR estimates for IMPT and h-IMRT treatment plans were 54.26 per 10,000 PY and 212.61 per 10,000 PY, respectively. Hence the LAR estimates from the IMPT plans are at least a factor of four times smaller than that of the h-IMRT plans. This trend is observed for all other sites, with the ipsilateral lung having the highest LAR estimate, followed by the contralateral breast and then the contralateral lung. This suggests that the breast patients undergoing IMPT have a reduced risk of developing secondary carcinogenesis, compared to those undergoing h-IMRT.
Table 5. Summary tables for the mean risk estimates with the corresponding ranges when the BEIR VII risk model [Citation13] was used to calculate: (a) the excess relative risk, (b) the excess absolute risk, and (c) the lifetime attributed risk.
The risk estimates calculated using the Schneider et al. [Citation15] model are presented in . Once again, the mean risk estimates for the IMPT treatment plan were found to be lower than those of h-IMRT. In the case of the ipsilateral lung, the LAR estimates for the IMPT and h-IMRT treatment plans were 42.78 per 10,000 PY and 83.88 per 10,000 PY, respectively. Therefore, for the Schneider et al. model, a factor of 2 was estimated between the IMPT and h-IMRT risks. These results agree with the BEIR VII risk estimates, which show a reduced risk of secondary carcinogenesis for IMPT.
Table 6. Summary tables for the mean risk estimates with the corresponding ranges when the Schneider et al. [Citation15] risk model was used to calculate: (a) the excess relative risk, (b) the excess absolute risk, and (c) the lifetime attributed risk.
Discussion
In general, the risk estimates from the Schneider et al. model were lower than those from the BEIR VII model. This is likely attributed to the fact that the Schneider et al. model assumes a reduced risk at higher doses due to cell death caused by irradiation [Citation18]. This finding also matches previous research conducted by Johansen et al. [Citation8], where reduced risk estimates were observed amongst all treatment plans for the non-linear risk model.
Overall the Schneider et al. model may be the more reliable for estimating risk as it contains information on cell death/repopulation effects [Citation15,Citation18]. It also uses data for patients who were exposed to higher doses that is more akin to the maximum doses in this research [Citation19]. While the BEIR VII report is not recommended to be used for high doses as in radiotherapy, the BEIR VII model is still significant, as it serves as a potential overestimate for the risk involved in a particular treatment plan.
Santos et al. [Citation7] have previously estimated LAR for breast cancer patients treated with radiotherapy. The treatment plans considered in this study were whole breast treatment, segmented breast treatment, accelerated partial breast irradiation, and MammoSite treatment, of all which utilise X-rays. Here, the IMPT LAR estimate for the ipsilateral lung was found to be less than half of the corresponding LAR estimates for the treatment plans listed above. The LAR estimates for the other organs were also observed to be lowest for IMPT.
Recently, De Rose et al. [Citation10] and Paganetti et al. [Citation11] have estimated EAR for breast cancer patients receiving comprehensive nodal irradiation, and included proton beam therapy treatment plans. These proton therapy plans were compared to IMAT and to conventional photon techniques. Proton beam therapy was estimated to have lower LAR than the conventional photon technique. However, the highest risks were associated with the IMAT treatment technique.
In calculating the LAR estimates, an age of exposure of 30 years was used for all patients. The reason for this is that younger patients are more susceptible to developing SPC when undergoing radiation treatment [Citation6,Citation20]. Thus, for patients undergoing breast treatment whose age is greater than 30 years, the LAR estimates presented here serve as an overestimate to the true risks. It should be noted however, that the patients considered in this study were likely over 30 years of age. Although this has an effect on the results in the study, the LAR estimates for IMPT are likely to still be less than that of h-IMRT as the age of exposure was constant between the two treatment plans.
The patient scans used in this study were in DIBH. While it has been shown that DIBH does not provide a significant dosimetric benefit for proton beam therapy, there are benefits for the photon treatment plans of left-sided breast patients [Citation21]. Therefore, the DIBH patient setup was chosen to provide the optimal photon plan in the comparison of the two treatment techniques.
A limitation of this study is that the dose to the skin cannot be accurately measured by the TPS, and was not taken into account in this study. However, future planning studies should adopt a technique similar to Depauw et al. [Citation22], where the dose to the 3–5 mm area between the skin and the PTV is limited during the plan optimisation.
While the risk of heart disease was not evaluated in this study, it is a major issue for breast cancer patients receiving radiotherapy [Citation23]. International Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guidelines state for partial heart irradiation a V25Gy <10% will be associated with a < 1% probability of cardiac mortality in long-term follow-up after RT [Citation24]. shows that this was met in all plans; however, a significant reduction was observed for the IMPT plans.
Conclusion
The results of this study show a reduced LAR estimates for IMPT compared to those from h-IMRT. This observation was consistent amongst the two risk models for both lungs and the contralateral breast. For both treatment techniques, the organ with the highest LAR estimate was the ipsilateral lung. This is also consistent with previous research conducted. Overall, these results suggest that the use of protons for breast treatment leads to reduced risk estimates for secondary carcinogenesis.
Disclosure statement
The authors report no conflicts of interest. No funding has been received for this work.
References
- Australian Institute of Health and Welfare. Cancer in Australia 2019. Canberra: AIHW; 2019.
- Sam B, Gunes G, David CW, et al. Mutational signatures of ionizing radiation in second malignancies. Nat Commun. 2016;7(1):12605.
- Piotrowski I, Kulcenty K, Suchorska W, et al. Carcinogenesis induced by low-dose radiation. Radiol Oncol. 2017;51(4):369–377.
- Marcu LG, Santos A, Bezak E. Risk of second primary cancer after breast cancer treatment. Eur J Cancer Care (Engl). 2014;23(1):51–64.
- Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol. 2015;114(1):56–65.
- Donovan EM, James H, Bonora M, et al. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys. 2012;39(10):5814–5824.
- Santos AMC, Marcu LG, Wong CM, et al. Risk estimation of second primary cancers after breast radiotherapy. Acta Oncol. 2016;55(11):1331–1337.
- Johansen S, Danielsen T, Rune Olsen D. Estimated risk for secondary cancer in the contra-lateral breast following radiation therapy of breast cancer. Acta Oncol. 2008;47(3):391–396.
- Corbin KS, Mutter RW. Proton therapy for breast cancer: progress & pitfalls. Breast Cancer Management. 2018;7(1):BMT06.
- De Rose F, Cozzi L, Meattini I, et al. The potential role of intensity-modulated proton therapy in the regional nodal irradiation of breast cancer: a treatment planning study. Clin Oncol (R Coll Radiol). 2020;32(1):26–34.
- Paganetti H, Depauw N, Johnson A, et al. The risk for developing a secondary cancer after breast radiation therapy: comparison of photon and proton techniques. Radiother Oncol. 2020;149:212–218.
- Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol. 2002;62(2):137–145.
- National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII, phase 2. Washington (DC): National Academies Press; 2006.
- Australian Bureau of Statistics. Life tables, Australia; 2004.
- Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Model. 2011;8(1):27.
- Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168(1):1–64.
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37(2–4):1.
- Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy using the linear-quadratic formula . Med Phys. 2009;36(4):1138–1143.
- Dores GM, Metayer C, Curtis RE. Second malignant neoplasms among long-term survivors of Hodgkinʼs disease: a population-based evaluation over 25 years. Oncol Times. 2002;24(12):89–90.
- Hankey BF, Curtis RE, Naughton MD, et al. A retrospective cohort analysis of second breast cancer risk for primary breast cancer patients with an assessment of the effect of radiation therapy. J Natl Cancer Inst. 1983;70(5):797–804.
- Patel SA, Lu H-M, Nyamwanda JA, et al. Postmastectomy radiation therapy technique and cardiopulmonary sparing: a dosimetric comparative analysis between photons and protons with free breathing versus deep inspiration breath hold. Pract Radiat Oncol. 2017;7(6):e377–e384.
- Depauw N, Batin E, Daartz J, et al. A novel approach to postmastectomy radiation therapy using scanned proton beams. Int J Radiat Oncol Biol Phys. 2015;91(2):427–434.
- Aznar MC, Korreman S-S, Pedersen AN, et al. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol. 2011;84(1004):743–746.
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose–volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl.):S77–S85.
