Abstract
Background/Purpose
The optimal dose fractionation for palliative radiotherapy (RT) in patients with symptomatic advanced bladder cancer is unclear. This study aimed to determine if a higher dose of RT was associated with improved symptoms response rates.
Methods
We searched PubMed, Central and Embase for eligible studies published from 1990 to 2019. The primary outcomes were symptoms response rates for hematuria, dysuria and frequency. Secondary outcomes included treatment-related adverse events and quality of life.
Results
We found one randomized, four prospective and eight retrospective non-comparative observational studies including 1320 patients who received palliative bladder radiotherapy for symptom relief. The dose fractionation schedules varied across studies ranging from 8 Gy single fraction to 60 Gy in 2 to 8 Gy per fraction. The pooled response rates for hematuria, dyuria and frequency symptoms were 74%, 58% and 71% respectively. A higher dose of RT was not associated with improved response rates of hematuria and frequency. However, a higher dose of RT was associated with a longer duration of hematuria response and reduced response of dysuria. Grade 3 gastrointestinal and genitourinary toxicity occurred in up to 26% of patients. Health-related quality of life (HRQOL) outcomes were reported in one study.
Conclusion
This systematic review demonstrates that a higher dose of bladder RT was not associated with improved response rates of hematuria and frequency symptoms but was associated with reduced response of dysuria. Higher doses of bladder RT was associated with more durable hematuria response. Prospective studies to determine the effects of palliative bladder radiotherapy on HRQOL outcomes are warranted
Introduction
Bladder cancer accounts for 549 393 new cases and 199 922 deaths worldwide in 2018, affecting primarily the elderly with the median age of diagnosis of 69 years old for men and 71 years old for women [Citation1]. Patients with bladder cancer can present with painless hematuria, with or without irritative voiding symptoms such as dysuria, frequency.
Palliative bladder radiotherapy for patients with bladder cancer is commonly used for palliation of local symptoms such as bleeding, dysuria and frequency. Hypofractionated regimens i.e., more than 2 Gy per fraction are usually preferred for the convenience of patients. Currently, the BA09 multicenter trial is the only randomized trial comparing two different hypofractionated regimens (35 Gy in 10 fractions versus 21 Gy in 3 fractions) for palliative treatment of local symptoms related to advanced bladder cancer [Citation2]. This randomized trial demonstrated that there was no statistically significant difference in symptom improvement or overall survival between these two arms. However, it is unclear what the optimal hypofractionated dose regimen is as only two fractionation regimens were compared in this trial. It is also unclear if duration of symptoms response is longer with higher biologically effective dose (BED). To date, several primary studies have been published on the efficacy and safety of palliative bladder RT in treating the local symptoms of advanced bladder cancer [Citation2–13]. To our knowledge, no review has been done to systematically evaluate the outcomes and side effects of this intervention. Thus, the primary aim of this systematic review was to summarize the current literature to determine the optimal dose/fractionation regimen for control of local symptoms in patients with bladder cancer. This study also aimed to evaluate the effects of palliative bladder radiation therapy on the toxicity and health related quality of life outcomes.
Materials and methods
Search strategy
The search strings for PubMed, Central and Embase were devised by JT and YYS for relevant studies published between January 1990 to December 2019 (Supplementary Table 1). This was because we felt that radiotherapy techniques will be representative of current practice. The controlled vocabulary terms (i.e., Medical Subject Headings) and its synonyms related to the concepts on Bladder neoplasms, Radiotherapy and Palliative Care were used in the literature search. The search strategy was restricted to human clinical studies published in English. We did not search trial registries. The studies were imported to Covidence for deduplication and further selection. The titles/abstracts/full text were screened by two authors (JT, YYS) independently. Disagreements were resolved by consensus. Publications were included for full text screening if they reported the use of any palliative bladder radiation therapy for the treatment of bladder cancer.
Eligibility
Published studies of radiotherapy to the bladder for palliation of local symptoms were eligible. Studies that reported symptom response, toxicity or quality of life were included. All study designs except case-reports and reviews were included. Studies evaluating radiotherapy combined with other tumor-directed treatment modalities (except chemotherapy) or re-irradiation were excluded. If multiple studies were published by the same author or institution, the most updated dataset would be used.
Evaluation of studies
We adopted the Newcastle–Ottawa quality assessment scale for assessment of quality of studies in our systematic review [Citation14]. Potential articles were evaluated at the full text level by two authors and final selection was based on consensus.
Data extraction and management
Data regarding the study characteristics and outcomes of interest (symptom response, toxicity and HRQOL) were extracted from the included studies using standardized forms. Two reviewers (JT and YYS) performed data extraction independently and a third reviewer (BV) was consulted to resolve discrepancies.
Outcomes
The primary outcomes were proportion of patients who responded for hematuria, dysuria and frequency symptoms. Response was defined as any reduction in symptoms severity or frequency as per the primary study investigators within 3 months after initiation of palliative bladder RT. The main summary measures for the primary outcomes were defined as proportions of patients with response relative to the total number of patients in each specified symptom category (hematuria, dysuria and frequency). Secondary outcomes included symptoms recurrence rate post completion of bladder radiation therapy, measured as time from completion of bladder radiation therapy to symptoms recurrence, adverse events and quality of life.
Statistical analysis
We computed the 95% confidence intervals for each study’s summary measure for the primary outcomes using the exact binomial method [Citation15]. As the data were primarily from uncontrolled retrospective studies, a meta-analysis of proportions based on the random effects model using the Der Simonian and Laird method was used to calculate the overall pooled estimates for the primary outcomes [Citation16]. For the secondary outcome on symptom recurrence rate which was measured as time to event data, we combined the individual log transformed hazard ratios and their variances using the generic inverse variance method and random effects model using the Der Simonian and Laird method. Statistical heterogeneity of the combined results was assessed by the I2 statistic [Citation10]. An I2 value of lower than 25% was interpreted as signifying a low level of heterogeneity. The meta-analysis was performed using the metaprop and metan commands in STATA version 14 (Stata Corp., College Station, TX, USA). All p values were 2 tailed with significance set at p < .05.
Subgroup analysis
Subgroup analyses were performed to determine if the results were influenced by the Biological Effective dose (BED). To analyze for a dose response relationship for patients who presented with bleeding, the cut off BED of 36 Gy, corresponding to the commonly prescribed dose fractionation regimen of 21 Gy in 3 fractions used to palliate localized bladder symptoms was used.
The BED is an approximate quantity by which different RT fractionation regimens are compared. It is given by BED = nD(1 + [D/{α/β}]), where n = number of fractions D = dose/fraction, nD = total dose, and α/β is the alpha/beta ratio, and is taken to be 10 for urothelial carcinomas.
Where the studies reported outcomes according to BED, they were grouped into A: BED ≥36Gy and B: BED <36Gy.
Meta Regression of the studies using BED as a covariate was performed using Der Simonian and Laird random effects model. In addition, sensitivity analysis was performed to evaluate the effect of BED according to the design of the study (prospective vs. retrospective).
We adopted the Synthesis without meta-analysis (SWiM) guidelines for the reporting of the secondary outcomes i.e., adverse events and HRQOL [Citation17]. Briefly, the SWiM guidelines cover the reporting on how studies are grouped, the standardized metric used for the synthesis, the synthesis method, how data are presented and a summary of the synthesis findings.
Results
Search results
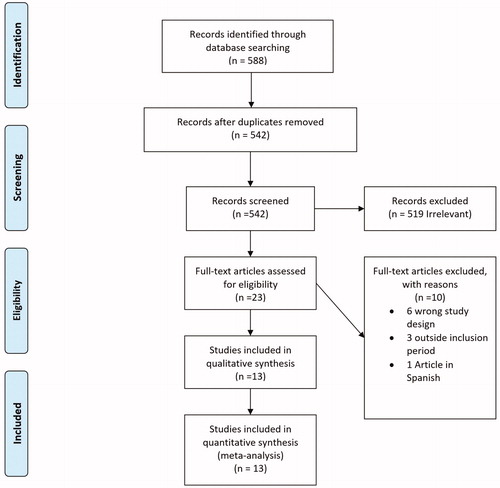
The results of the study selection process are outlined in . We identified a total of 588 studies. After removing 46 duplicates and excluding 519 irrelevant studies, 23 studies were assessed for eligibility. After applying the eligibility criteria, 10 studies were excluded: 6 studies had the wrong study design, 3 were outside the inclusion period and one article was in Spanish. 13 studies including 1320 patients who received palliative radiotherapy for relief of local symptoms were included in the final quantitative analysis.
Patient characteristics and symptoms
summarizes the characteristics of the studies. The studies were published from 1990 to 2019. We found one randomized study [Citation2], 4 prospective phase 2 studies [Citation3,Citation6–8] and eight retrospective non-comparative observational studies [Citation4,Citation5,Citation9–11,Citation13,Citation18,Citation19]. The sample size of the included studies ranged from 14 to 500 patients. Seventy three percent (687/942) of patients were male. Median age of patients reported in the included studies was 80 years (range 76–82 years). Median follow-up of patients reported in the included studies was 21 months (range 3.5–29 months). All patients had urothelial carcinoma and all patients received radiotherapy for palliation of local symptoms. All patients in the studies had hematuria as the presenting symptom Five studies included patients who presented with dysuria [Citation2,Citation4,Citation6–8] and 3 studies included patients who presented with frequency [Citation2,Citation6,Citation8]. Three studies included patients with both locally advanced disease [Citation4,Citation7,Citation8] and the remaining 10 studies included patients with both locally advanced and metastatic disease [Citation2,Citation3,Citation5,Citation6,Citation9–13,Citation19].
Table 1. Characteristics of studies of palliative radiotherapy for Bladder Cancer.
Methodological quality of included studies
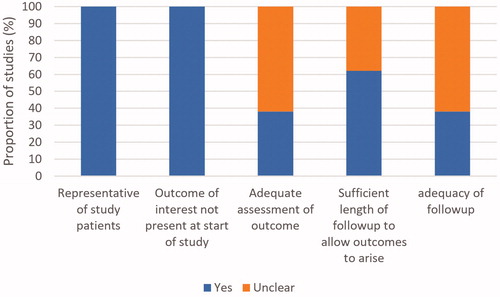
The methodological quality of included studies is summarized in . All studies enrolled a representative sample of patients and outcomes of interest were not present at the start of the study. Adequate assessment of response was not clear in eight studies [Citation4,Citation5,Citation9–13,Citation19]. Sufficient length of follow-up to allow outcomes to arise was not clear in five studies [Citation2–5,Citation8]. Follow-up adequacy was unclear in eight studies [Citation2–5,Citation8,Citation12,Citation13].
Radiotherapy dose, fractionation and target and technique
The dose fractionation schedules varied between, and often within studies. Fraction sizes ranged from 2 to 8 Gy and total doses ranged from 8 Gy to 60 Gy. The BED ranged from 14.4 Gy to 72 Gy. No patient received concurrent chemoradiotherapy. Five studies planned patients using 3 dimensional imaging [Citation4,Citation6,Citation8,Citation9,Citation11], Three studies used both 2 and 3-dimensional imaging [Citation7,Citation12,Citation19]. Four studies did not report the planning technique used [Citation2,Citation3,Citation10,Citation13]. Target volumes definitions for radiotherapy were variable. Majority of studies included the bladder as the Clinical Target Volume (CTV). In addition, one study treated the pelvic nodes [Citation10]. The margins from CTV to the Planning Target Volume (PTV) ranged from 1–2 cm. All studies except one (supervoltage therapy) [Citation4] treated patients with high energy megavoltage photons (6–16MV). Only one study provided dose constraints used for radiotherapy planning [Citation8]. Common field arrangements used included Anterior-Posterior (AP)/Posterior-Anterior fields (PA), three fields (AP and two posterior obliques) and four fields (AP/PA and 2 parallel opposed laterals).
Treatment response
Response criteria varied across studies for hematuria, dysuria and frequency. Symptom response was assessed at different time points in the included studies. The time point used (number of studies) are as follows: End of RT (1) [Citation3], one week after completion of RT(1) [Citation19], two weeks after completion of RT (1) [Citation9], three weeks after RT (1) [Citation10], one month after completion of RT (1) [Citation6], six weeks after completion RT (1) [Citation13], two months after completion of RT (1) [Citation11] and three months after completion RT (4) [Citation2,Citation4,Citation7,Citation8]. Two studies did not mention the time of assessment of response [Citation5,Citation12].
Hematuria
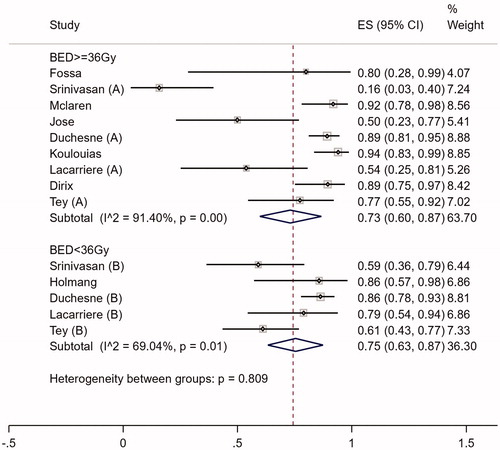
All 13 studies included 1320 patients who presented with hematuria. The definition of symptom response varied between studies. Proportion of patients who responded for hematuria ranged from 39% to 94% (). The median duration of response reported ranged from 3.6 to 14 months. Nine hundred and sixty-six patients were eligible for subgroup analyses as BED data were available. The overall pooled proportion of patients who responded for hematuria was 74% (95% CI 0.66–0.83. I2=86%) (Supplementary Figure 1).
Table 2. Symptom response to palliative bladder therapy.
Subgroup analysis showed that there was no evidence supporting a difference in response for bleeding between regimens with BED ≥36Gy vs. regimens with BED < 36Gy (Chi2=0.809) (). The metaregression model including BED as a covariate showed that increasing BED was not associated with higher bleeding response (p = .435) (Supplementary Figure 2).
Dysuria
Five studies included a total of 232 patients who presented with dysuria. Two studies used a 3-point scale to grade symptom response [Citation4,Citation7]. One study used the MRC BA09 bladder and bowel symptom grading system and two studies defined ‘improvement of dysuria’ as a response [Citation8,Citation13]. Proportion of patients who responded for dysuria ranged from 44% to 72% (). Median duration of response for dysuria was not reported specifically in the studies. The overall pooled proportion of patients who responded for dysuria is 58% (95% CI 0.47−0.70. I2 =69.4, p < .05%) (Supplementary Figure 3).
Subgroup analysis showed that there was no evidence supporting a difference in response for dysuria between regimens with BED ≥36Gy vs. regimens with BED < 36Gy (Chi2=0.133) (Supplementary Figure 4). The metaregression model including BED as a covariate showed that increasing BED was associated with decreased dysuria response (p = .012) (Supplementary Figure 5).
Frequency
Three studies included a total of 94 patients who presented with frequency [Citation2,Citation7,Citation8]. One study used a 3-point scale to grade symptom response [Citation7], one study used the MRC BA09 bladder and bowel symptom and toxicity grading system [Citation2] and one study defined ‘improvement of frequency’ as a response [Citation8]. Proportion of patients who responded for frequency ranged from 57% to 88% (). Median duration of response for frequency was not specifically reported in the studies. The overall pooled proportion of patients who responded for frequency was 71% (95% CI 0.56–0.87. I2 =67.53%, p < .05) (Supplementary Figure 6).
Subgroup analysis showed that there was no evidence supporting a difference in response for frequency between regimens with BED ≥ 36 Gy vs. regimens with BED < 36Gy (Chi2=0.646) (Supplementary Figure 7). The metaregression model including BED as a covariate showed that increasing BED was not associated with increased frequency response (p = .267) (Supplementary Figure 8).
Symptoms recurrence
Only two studies reported hematuria recurrence in patients with an initial response to palliative bladder radiotherapy [Citation12,Citation19]. One study reported that increasing BED was significantly associated with prolonged freedom from recurrent bleeding [Citation12]. Our group previously reported that patients treated with low BED regimens (defined as <36Gy BED10) had increased hazard of recurrence of bleeding compared with high BED (defined as ≥ 36 Gy BED10) regimens [Citation19]. Using the individual patient data of this study, we calculated the hazard ratio as per Aljabab et al. Meta-analysis of these two studies showed that for every one Gy increase in BED, there was an associated relative reduction in the hazard of rebleeding by 7%. (HR 0.93, 95% CI 0.89–0.98; p = .003), adjusted for gender and stage (Supplementary Figure 9).
Sensitivity analysis
Sensitivity analysis according to study design (prospective vs. retrospective) showed that there was no difference in bleeding and frequency response between low vs. high BED regimens. Similar findings were seen in dysuria response in prospective trials. However, dysuria response was significantly lower for higher BED regimens in retrospective studies (Supplementary Tables 2 and 3).
Toxicity
An overview of the toxicities reported in all studies is presented in . Seven of thirteen studies reported toxicity outcomes [Citation2,Citation5–8,Citation10,Citation11,Citation19]. Grading scales such as Radiation Therapy Oncology Group (RTOG) were used in 3 studies [Citation7,Citation8,Citation11], the Common Toxicity Criteria (CTC) scales were used in one study [Citation19], the MRC B09 bladder and bowel symptom and toxicity grading scale was used in one study [Citation6] and one study utilized investigator graded toxicity [Citation5]. Grade 3 gastrointestinal and genitourinary toxicity occurred in up to 26% of patients treated with palliative radiotherapy.
Table 3. Toxicity reported in studies of palliative radiotherapy for bladder cancer.
HRQOL
QOL data was reported in the BA09 randomized trial [Citation2]. Changes in QOL scores between pretreatment and 3 month assessments were reported. Briefly, there was no evidence of a difference in the change of any symptom between the two treatment arms (35 Gy/10 fractions vs. 21 Gy in 3 fractions) in this study. In addition, there was no evidence of a difference in the change between the pretreatment and end of treatment assessment between the two arms.
Discussion
To the best of our knowledge, this is the first systematic review that summarizes quantitatively both prospective and retrospective evidence examining the efficacy of different dose fractionation schedules of palliative bladder radiotherapy for localized bladder symptoms namely hematuria, dysuria and frequency. After a comprehensive literature search, we found thirteen relevant studies, including 5 prospective trials and 8 retrospective studies reporting outcomes of palliative radiotherapy for bladder cancer. Our review showed that radiotherapy for localized bladder cancer symptoms was associated with a high proportion of patients who respond, with pooled overall proportion of patients who respond for bleeding, dysuria and frequency symptoms of 74%, 58% and 71% respectively. This is consistent with palliative radiotherapy for other organ sites.
Although there was significant heterogeneity in the dose fractionation regimens used (ranging from 8 Gy to 60 Gy in 2–8Gy per fraction), the pooled overall proportion of patients who respond of ≥60% suggests that radiotherapy is effective in palliating localized bladder bleeding, dysuria and frequency. Pooled response for bleeding, dysuria and frequency according to BED showed that there was no difference in proportion of patients who respond between regimens with BED of ≥ 36 Gy versus regimens with BED < 36Gy. The use of palliative RT for other tumor sites have yielded similar results. A systematic review of palliative gastric radiotherapy showed that there was no difference between high (≥39Gy) BED vs. low (<39Gy) BED regimens) for the palliation of gastric bleeding, pain and obstruction [Citation20]. Similarly, a systematic review of palliative thoracic radiotherapy for lung cancer showed that there was no difference in the palliation of hemoptysis, chest pain and cough between low vs high BED regimens (cut off BED 35 Gy, α/β = 10) [Citation21].
Interestingly, in employing palliative bladder RT for dysuria, the metaregression model including BED as a covariate showed that increasing BED was associated with decreased proportion of patients who respond (p = .012). This suggests that high dose RT is not desirable when palliating dysuria. This may be because higher doses of RT can cause increased bladder inflammation, leading to increased pain. However, the decrease in dysuria response was only seen in retrospective trials on sensitivity analysis based on study design. Further prospective trials are required to determine the optimal dose fractionation regimens for relief of dysuria.
Whilst there may be no difference in the proportion of patients who respond, our results suggest that the recurrence rates of hematuria may be lower with higher BED regimens compared with lower BED regimens. Meta-analysis of the studies reporting the association of BED with bleeding recurrence showed that for every one Gy increase in BED, there was an associated relative reduction in the hazard of rebleeding by 7%. (HR 0.93, 95% CI 0.89–0.98; p = .003), adjusted for gender and stage. This suggests that a higher BED regimen should be prescribed whenever possible as this may decrease emergency visits and admissions for treatment of recurrent hematuria, improving the patient’s quality of life. In addition, it may be cost effective due to avoidance of retreatment and hospital stay. A benefit for high dose RT in reducing retreatments was also seen in lung cancer. Fairchild et al showed that for palliative lung cancer treatments, the likelihood of reirradiation was 1.2-fold higher after low dose RT compared to high dose RT [Citation21].
Validated grading scales such as RTOG or CTC were used in the studies for toxicity assessment. Grade 3 gastrointestinal toxicity and bladder irritability occurred in up to 26% of patients. The relatively high rates of grade 3 toxicity may partly be explained by the fact that CT planning was performed in less than 50% of included studies. In addition to toxicity grading scales, patient reported outcomes (PROs) are also important in assessing toxicities from palliative treatments. In the studies assessed in our review, PROs were not reported in all but one study. PROs should be included in prospective studies of palliative radiotherapy as they allow measurement and meaningful comparison of side effects.
Whilst this review showed a benefit for palliative radiotherapy for localized bladder cancer symptoms, the reviewers also acknowledge that the included studies had several limitations. Firstly, a wide range of dose-fractionation regimens were used with varying definitions of response to radiotherapy for bleeding, dysuria and frequency, as well as different time points for assessment of treatment response. This precludes the conclusions of most appropriate dose fractionation regimens or dose response. Secondly, in non-randomized studies of palliative radiotherapy, the selection of RT dose-fractionation regimen may be based on the patient's performance status and estimated life expectancy and thus may lead to selection bias. Thirdly, the comparison of dose-response was performed across studies rather than within studies leading to potential bias in estimates of outcomes. Lastly, only one study reported PROs, which are important measures of the effects of palliative treatment and should be routinely included in studies of palliative interventions.
Conclusion
This systematic review demonstrates that a higher dose of bladder RT was not associated with increased proportion of response in patients with hematuria and frequency symptoms but was associated with reduced response of dysuria. Whilst Low dose regimens appear to be adequate for symptom palliation, high dose regimens may be preferred for durable control of hematuria. Prospective studies to determine the effects of palliative bladder radiotherapy on HRQOL outcomes are warranted
Author contributions
Dr Tey is responsible for conceptualization of the study. Dr Tey and Dr Soon are responsible for data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, writing the original draft and review and editing the manuscript. Dr Ho, Dr Koh, Dr Chia, Dr Ooi, Dr Tuan, Dr Vellayappan are responsible for investigation, methodology, project administration, resources, writing the original draft and reviewing and editing the manuscript
Supplemental Material
Download Zip (2.9 MB)Supplemental Material
Download PDF (634.5 KB)Disclosure statement
All the authors have no conflict of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379–388.
- Fossa SD, Hosbach G. Short-term moderate-dose pelvic radiotherapy of advanced bladder carcinoma. A questionnaire-based evaluation of its symptomatic effect. Acta Oncol. 1991;30(6):735–738.
- Srinivasan V, Brown CH, Turner AG. A comparison of two radiotherapy regimens for the treatment of symptoms from advanced bladder cancer. Clin Oncol (R Coll Radiol)). 1994;6(1):11–13.
- Holmang S, Borghede G. Early complications and survival following short-term palliative radiotherapy in invasive bladder carcinoma. J Urol. 1996;155(1):100–102.
- McLaren DB, Morrey D, Mason MD. Hypofractionated radiotherapy for muscle invasive bladder cancer in the elderly. Radiother Oncol. 1997;43(2):171–174.
- Jose CC, Price A, Norman A, et al. Hypofractionated radiotherapy for patients with carcinoma of the bladder. Clin Oncol (R Coll Radiol). 1999;11(5):330–333.
- Kouloulias V, Tolia M, Kolliarakis N, et al. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. Int Braz j Urol. 2013;39(1):77–82.
- Lacarriere E, Smaali C, Benyoucef A, et al. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol. 2013;39(6):808–816.
- Mery B, Falk AT, Assouline A, et al. Hypofractionated radiation therapy for treatment of bladder carcinoma in patients aged 90 years and more: a new paradigm to be explored? Int Urol Nephrol. 2015;47(7):1129–1134.
- Dirix P, Vingerhoedt S, Joniau S, et al. Hypofractionated palliative radiotherapy for bladder cancer. Support Care Cancer. 2016;24(1):181–186.
- Aljabab S, Cheung P, Dennis K, et al. Hemostatic radiotherapy in advanced bladder cancer: a single-institution experience. J Radiat Oncol. 2017;6(4):379–385.
- Ali A, Song YP, Mehta S, et al. Palliative radiation therapy in bladder cancer-importance of patient selection: a retrospective multicenter study. Int J Radiat Oncol Biol Phys. 2019;105(2):389–393.
- Wells GS, O’Connell D, et al. The Newcastle-Ottawa Scale for assessing the quality of non-randomized studies in meta-analyses. [cited 2021 Jan 19]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890:
- Aljabab S, Cheung P, Dennis K, et al. Hemostatic radiation therapy in advanced bladder cancer: a single-institution review. Int J Radiat Oncol Biol Phys. 2014;90(1):S696.
- Tey J, Soon YY, Cheo T, et al. Efficacy of palliative bladder radiotherapy for hematuria in advanced bladder cancer using contemporary radiotherapy techniques. In Vivo. 2019;33(6):2161–2167.
- Tey J, Soon YY, Koh WY, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(15):25797–25805.
- Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001–4011.