Introduction
Administration of immune checkpoint inhibitors (ICIs) induces different patterns of response, including the classical complete or partial response and disease progression and new patterns such as hyperprogressionand pseudoprogression. Pseudoprogression is defined as an increase in tumor size followed by a response to treatment, resulting from an exacerbated immune cell infiltration in the tumor bed, including CD103+ and CD8+ cells [Citation1]. Pseudoprogression is a rare phenomenon, with a rate not exceeding 10% in patients treated with ICI [Citation2]. In everyday practice, the misclassification of pseudoprogression as disease progression remains a concern. According to iRECIST guidelines, confirmation of the progression with later imaging is mandatory to make sure that the 'unconfirmed' progression is 'disease progression' and not 'pseudoprogression' [Citation3]. Hodi et al. [Citation4] described that pseudoprogression occurs more often at the beginning of ICI treatment. Here, we report two cases of dramatic pseudoprogression occurring 36 and 10 months after the initiation of ICI.
Case reports
Case report 1
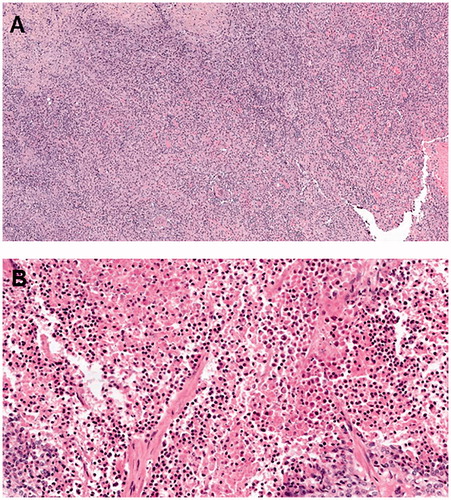
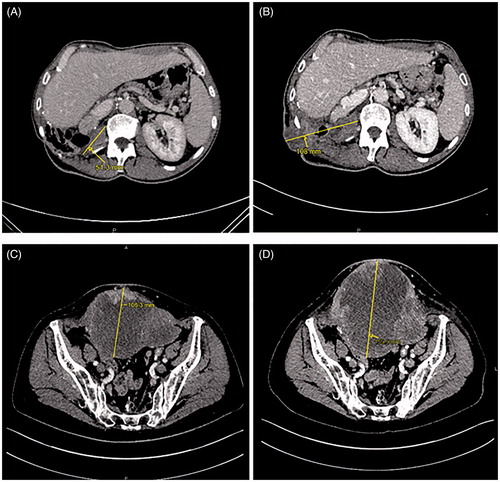
A 44-year-old man with no medical history was diagnosed with high-grade renal cell carcinoma in 2011. He was initially treated with right radical nephrectomy. Six months later, a local recurrence was treated by surgery. In 2012, a new local recurrence was treated with sunitinib. In 2013, because of disease progression (appearance of one liver metastasis), the patient received second-line treatment with sorafenib. A few months later, because of stable disease, the patient underwent hepatectomy and resection of the local recurrence. Systemic treatments were then discontinued until April 2014, when pancreatic metastasis was diagnosed. Due to stable disease, the patient underwent a duodenopancreatectomy in August 2014. Two more surgical procedures were performed in February 2015 and May 2016 for the management of local relapse in the primary surgical bed. In March 2017, multiple bilateral lung metastases were diagnosed, and treatment was initiated with an ICI (nivolumab 3 mg/kg every 2 weeks). In May 2017, after a seizure, brain metastasis was diagnosed and treated with stereotaxic radiotherapy. Nivolumab was then resumed. The patient experienced a partial response and good quality of life. In October 2018, progression was found in lung metastases and treated with stereotaxic radiotherapy. Nivolumab was then resumed. A second pulmonary progression was found in November 2019 and required stereotaxic surgery. From March 2017 to March 2020, the patient received 70 cycles of nivolumab. In April 2020, the patient described loss of appetite, asthenia, pruritus, and pain in the right flank. A computed tomography (CT) scan showed a necrotic mass in the primary surgical bed, and this was consistent with a local relapse (). However, a few days later, a cutaneous fistula was observed in the right flank with a risk of colonic fistula, requiring an emergency surgical procedure. There was no fever, and the C-reactive protein level was within the normal range. The surgical specimen contained only necrotic tissue with immune cell infiltration (chronic inflammatory lymphocytic and macrophagic infiltrate), and there was no residual tumor cell (). These findings were consistent with pseudoprogression. After the surgery, the patient healed slowly and was in a good condition. In November 2020, we observed progression of lung metastases without local relapse.
Figure 1. An increase in the size of a necrotic mass in the surgical bed of the primary tumor with cutaneous fistula in the right flank on computed tomography (CT) scan for restaging in April 2020 (B) compared to that in January 2020 (A) in case 1, and an increase in the size of a necrotic peritoneal carcinomatous mass with cutaneous infiltration in October 2017 (D) compared to that in January 2017 (C) in case 2.
Case report 2
A 74-year-old woman with medical history of hypertension and hypothyroidism was diagnosed with high-grade endometrial adenocarcinoma in 2014. She was initially treated with total abdominal hysterectomy and bilateral salpingo-oophorectomy, four cycles of adjuvant chemotherapy (carboplatin–paclitaxel) and pelvic radiotherapy. At the end of the adjuvant radiotherapy, adrenal and peritoneal metastases were diagnosed and treated with subsequent lines of chemotherapy (liposomal doxorubicin, then weekly paclitaxel, then weekly epirubicin) without clinical benefit. In July 2016, the mismatch repair (MMR) test showed the microsatellite instability-high (MSI-H) phenotype, leading to the initiation of treatment with an ICI (pembrolizumab 200 mg every 3 weeks). At the time, the extent of the disease was diffuse peritoneal metastases and massive pelvic and retroperitoneal lymph nodes. After initiation of therapy, the best response was shrinkage of diffuse peritoneal metastases and retroperitoneal lymph nodes and stable disease of the peritoneal mass. However, her general condition improved with a good quality of life. In May 2017, after 17 cycles of pembrolizumab, the patient experienced severe pelvic pain and the pelvic skin was inflamed. The peritoneal mass was found to be necrotic, with a risk of skin fistula. C-reactive protein level was abnormal, twice the upper limit of normal. CT scan showed a slight increase in pelvic mass size (179 mm compared to 134 mm in January 2017) (). The patient underwent palliative surgery with the removal of this mass in May 2017. The surgical specimen contained chronic inflammatory lymphocytic infiltration surrounding undifferentiated adenocarcinoma cells in the peritoneal mass and complete pathological response in the lymph nodes. (). These findings were consistent with pseudoprogression. Pembrolizumab was discontinued. CT scans in November 2017 and January 2018 demonstrated complete response. This complete response was maintained until October 2020 without further treatment.
Discussion
We here report for the first time two cases of late-onset life-threatening pseudoprogression requiring emergency surgery with histopathology of the surgical specimens. In both cases, pseudoprogression occurred after several months of ICI treatment (36 and 10). The pseudoprogressions were symptomatic with pain and inflamed skin. There was no fever. The pseudoprogressions occurred in the surgical bed of the primary tumor. At the metastatic sites, we observed stable disease (case 1) or partial response (case 2). In both cases, there was a slight increase in the size of the primary site tumor. CT scans did not find specific patterns suggesting pseudoprogression, excluding the fact that both masses were necrotic. In both cases, we observed symptomatic pseudoprogressions requiring emergency surgery to avoid fistulation. The risk-benefit ratio of surgery in the context of heavily pretreated, advanced cases was discussed extensively with surgeons, anesthesiologists, patients, and families. In both cases, we discussed two opposite approaches: symptomatic surgery of pseudoprogression versus palliative care. Decision making had to be done under pressure, without a definitive diagnosis (progression versus pseudoprogression). Biopsies may have been helpful; however, with the risk of fistulation, biopsies of these necrotic masses were deemed to be inappropriate. In conclusion, only the pathological report confirmed the diagnosis of pseudoprogression. As previously reported [Citation5], pathological examination revealed that the increase in lesion size was likely due to massive lymphocytic infiltration in both cases, and complete pathological response without cancer cells was observed in one case.
Pseudoprogression is an atypical pattern of response. In some cases, it could be associated with dramatic symptoms, such as tamponade and pleural effusions [Citation6], intracranial hypertension [Citation7], or bowel perforation [Citation8]. As in our two cases, pseudoprogression may also be late-onset after ICI initiation [Citation9,Citation10]. The severity of some clinical presentations and the misdiagnosis of pseudoprogression as disease progression could lead to inappropriate decisions such as discontinuation of ICI, contraindication to local treatment for managing complications of pseudoprogression, or referral to palliative care for treatment [Citation11]. Recognizing pseudoprogression is of major importance since in most cases, it is followed by long-term disease control [Citation12]. Radiographic follow-up, biopsy, histologic examination of enlarged lesions, circulating tumor DNA, and serum interleukin-8 levels may help diagnose pseudoprogression [Citation13], underlining the need for a multidisciplinary approach for its management.
Conclusion
Physicians must be aware of the potential severe clinical presentation associated with pseudoprogression and the fact that new lesion appearance or growth of known lesions may not always signify a failure of disease control during ICI treatment. Indeed, as we observed in these two cases, pseudoprogression can occur after several months of ICI treatment.
Informed consent
Both patients have provided informed consent for publishing their medical history and personal medical data (M004; deliberation n° 2018-155 on the 3rd May 2018).
Supplemental Material
Download PDF (3.7 MB)Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing
Disclosure statement
No authors report any conflict of interest.
References
- Rocha P, Hardy-Werbin M, Naranjo D, et al. CD103 + CD8+ lymphocytes characterize the immune infiltration in a case with pseudoprogression in squamous NSCLC. J Thorac Oncol. 2018;13(10):e193–e196.
- Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30(3):385–396.
- Seymour L, Bogaerts J, Perrone A, RECIST working group, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152.
- Hodi FS, Hwu W-J, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517.
- Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44–48.
- Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy- a report of two cases. J Immunother Cancer. 2016;4:80
- Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4(3):179–182.
- Kim HK, Baek S-W, Jeong Y, et al. Pseudoprogression presenting as intestinal perforation in non-small cell lung cancer treated with anti-PD-1: A case report. Mol Clin Oncol. 2019;11(2):132–134.
- Suyanto S, Yeo D, Khan S. A rare delayed atypical pseudoprogression in nivolumab-treated non-small-cell lung cancer. Case Rep Oncol Med. 2019;2019:8356148
- Kumagai T, Kimura M, Inoue T, et al. Delayed pseudoprogression of lung adenocarcinoma accompanied with interstitial lung disease during chemotherapy after nivolumab treatment. Thorac Cancer. 2017;8(3):275–277.
- Wong AS, Thian Y-L, Kapur J, et al. Pushing the limits of immune-related response: a case of “extreme pseudoprogression". Cancer Immunol Immunother. 2018;67(7):1105–1111.
- Hochmair MJ, Schwab S, Burghuber OC, et al. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer patients treated with immunotherapy. Lung Cancer. 2017;113:4–6.
- Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16(4):655–670.