Abstract
Background
The extent to which positive surgical margins (PSM) affect the risk of subsequent salvage radiation therapy (sRT) or androgen depletion therapy (ADT) following radical prostatectomy (RP) is not well described. Initiation of additional therapies after RP depend on patient preference, individual factors, local guidelines, and life expectancy. The aim of this study was to analyze differences between margin status in risk of subsequent treatment for PCa following RP in a retrospective population-based cohort from Denmark.
Methods
Patients who underwent RP were identified in The Danish Prostate Cancer Registry (DaPCaR). Subsequent sRT and ADT were assessed in uni- and multivariate settings and validated with receiver operating characteristic (ROC).
Results
PSM was associated with an increased risk of sRT (HR = 1.85, p < .001) and receiving ADT (HR:1.39, p = .007). Margin status only had a minor impact on the predictive ability for sRT (area under the curve (AUC): p < .001) and no significant impact for subsequent ADT (AUC: p = 1). Significant inter-institutional difference in the association between PSM with sRT or ADT was observed.
Conclusion
PSM is associated with the risk of sRT and initiation of ADT, however this association is weak. Our results underline that factors beyond tumor characteristics play a major role for initiation of sRT and ADT.
Introduction
The definition of a positive surgical margin (PSM) and its effect on outcome after radical prostatectomy (RP) for prostate cancer (PCa) has been discussed over the years. A PSM is a microscopic diagnosis and many definitions have been proposed. Today, the most widely recognized definition is proposed by The International Society of Urological Pathology (ISUP) defining a PSM as any cancer cell(s) touching the inked margin of the prostate specimen [Citation1].
The risk of a PSM depends on several cancer related factors as clinical stage, pre-operative PSA, tumor burden, and biopsy Gleason score, but also surgical factors such as surgical experience, volume, and technique [Citation2–4]. Furthermore, studies have demonstrated interobserver variation among pathologists when assessing margin status [Citation5,Citation6].
Many regard a PSM as a marker of surgical quality, especially in localized pT2 tumors where a PSM may be the result of resection too close to the prostate. However, due to the disparity in definitions of PSM and differences in patient populations, caution should be made when comparing PSM rates across institutions or surgeons. Nevertheless, meta-analyses have demonstrated PSM to be a significant independent risk factor for biochemical recurrence (BR) in multivariate analysis. Overall, a PSM doubles the risk of BR compared to a negative surgical margin (NSM) [Citation7–9]. However, it is important to recognize that the individual absolute risk of BR may be low and is affected by the length of follow-up without recurrence, and dynamic prediction models should be used to assess the risk of BR for the individual patient, including for men with PSM [Citation10,Citation11]. Furthermore, BR is a surrogate marker for prognosis after RP and the long-term impact is less understood. The Scandinavian Prostate Cancer Group-4 study (watchful waiting vs. RP) did not find PSM to be associated with neither metastasis-free- nor PCa-survival [Citation12]. Conversely, a meta-analysis of 32 database suggests that PSM have an impact on cause-specific mortality (HR = 1.23, 95%CI: 1.16–1.30) compared to NSM [Citation13].
To what extent margin status affects the risk of subsequent therapy following RP and how this differs per institution is not well described. Salvage radiotherapy remains a secondary curative option for men with BR after RP, while androgen deprivation therapy (ADT) is the primary option for men with distant failure. As initiation of such additional therapies after RP depends on patient preference, individual factors, local guidelines, and life expectancy, it is important to address whether PSM affects the chance of receiving additional therapy when considering tumor-related factors and whether this differs per institution.
The aim of this study was to analyze risk of subsequent treatment following RP in a retrospective population-based cohort from Denmark and study the impact of PSM, tumor characteristics and institutional differences in treatment patterns.
Methods
All patients who underwent RP in Denmark from 1 January 1998 to 31 December 2011 were identified in the Danish prostate cancer registry (DaPCaR) [Citation14]. The database and study are approved by the Danish Health and Medicines Authority (file number: 3-3013-858/1/) and the ethical committee of The Capital Region of Denmark (protocol number: H4-2014-FSP). Data on subsequent therapies, salvage radiation and/or ADT was extracted from the National Patient Registry as approved by the Danish Data Protection Agency (file number: 2012-41-0390).
Patients without PCa in the specimen (pT0), missing information on hospital of care, margin status, age, Gleason grade or pathological tumor stage (pT-category) were excluded. Furthermore, we excluded men who received radiation or endocrine therapy within 3 months of RP, most of which participated in the RADICALS trial [Citation15]. Specifically, for multivariate analysis, all men with missing pre-operative PSA were excluded for the analysis (n = 2699).
All men have been treated according to national guidelines in one of five hospitals [Citation16]. According to guidelines, adjuvant radiation is not recommended in Denmark. If BR, defined as a rising PSA ≥0.2 ng/mL is confirmed, a patient should be assessed for additional therapy, sRT (33 fractions of 2 Gy with or without lymph node field) has been recommended for patients with BR, in whom local recurrence was suspected based on RP specimen characteristics, absolute PSA ≤0.7 ng/mL, and PSA kinetics (PSA doubling time (PSAdt) > 12 months). Adjuvant hormone therapy to sRT was at the treating physicians’ discretion. For the patients included in this study, guidelines proposed adjuvant ADT for up to 6 months; if a patient received ADT after 6 months, it was classified as permanent ADT from that moment onwards. None of the patients received 2 years of adjuvant Bicalutamide, as per RTOG 9601 study [Citation17]. Permanent ADT was recommended for men suspected for distant failure based on PSA kinetics (PSAdt <9 months), adverse prostate histopathology, level of PSA (>0.7 ng/mL) or men with metastasis on imaging.
Statistics
Descriptive analyses of continuous variables were reported as medians with interquartile ranges (IQR), categorical variables as numbers and percentages of total (%), and differences were assessed by Kruskal-Wallis Rank Sum Test or Analysis of Variance (ANOVA). Cumulative incidence of sRT and ADT was described using competing risk assessment with death before salvage treatment as a competing event and time from RP to either event or end of follow-up as underlining timescale. The risk of any event is presented as cumulative incidence curves stratified by margin status, with difference tested by Gray’s test. Median follow-up time was calculated by reverse Kaplan-Meier estimate. Multivariate modeling containing age, pre-operative PSA, GS, pT-category, lymph node status (N-category) and margin status was performed with cause-specific Cox proportional hazard model (CSC). Firstly, sRT, other first line treatment and death before treatment were competing events, secondly, ADT and death before treatment were treated as a competing event. The associations are reported as hazard ratios (HR) with corresponding 95% confidence intervals (CI). Sub-analysis was performed for pT2 and pT3 tumors. Proportional hazard assumptions of CSC were visually checked with Schoenfeld residual plots for proportionality. Model validation was performed by assessing the area under the curve (AUC) of the receiver operating characteristic (ROC) curve and prediction error (Brier score) [Citation18,Citation19]. The changes in the model for predicted risk of additional treatment by adding margin status to the multiple regression models were further assessed by reclassification diagrams and calibration plots for selected prediction horizons [Citation18]. Predicted events for sRT and mortality were calculated based on the CSC model [Citation20]. R v3.4.1 (R Development Core Team, Vienna, Austria) running on the Rstudio v1.0153 software (© 2009–2017 RStudio, Inc) was used for statistical analysis. Statistical significance was defined as a p-value of less than 0.05.
Results
A total of 5859 patients were included in the study, for detailed information see . Patient characteristics at RP are demonstrated in . The overall rate of PSM was 26.6%. The frequency of PSM varied significantly between institutions ranging from 20% to 53%. Furthermore, significant variations in baseline characteristics in biopsy Gleason grade and cT-category was found between institutions. Median follow up time was 8.12 years (IQR: 6.6–10.3). After 15 years, the cumulative incidence of PCa specific death was 7.9% (95CI: 6.1–9.8) and other causes of death 18.3% (95CI: 15.6–21.1). Univariate analysis demonstrated a significant difference (p < 0.001) in the cumulative incidence of PCa mortality for men with PSM (12.0%, 95CI: 8.4–15.6%) and NSM (6.0%, 95CI: 3.9–8.1%) after 15 years. This difference was not present among men who died from other causes (PSM:18.0%, 95CI: 14.7–21.3% vs. NSM 18.8%, 95CI: 14.1–23.5%, p = 0.38, respectively).
Figure 1. Flow chart of included patients. Abbreviations: RP: Radical prostatectomy; DaPCaR: Danish prostate cancer registry; N: number of subjects; PCa: Prostate cancer; pT-category: Pathological tumor category; PSA: Prostate-specific antigen.
Table 1. Baseline characteristics of patients at radical prostatectomy (RP) in total and stratified per hospital of care.
Salvage radiotherapy
Overall, the cumulative incidence of sRT was 13.1% (95CI: 12.2–13.9), 17.1% (95CI: 16.1–18.2), and 19.0% (95CI: 17.5–20.5) after 5, 10 and 15 years, respectively (). When stratified by margin status, the incidence of sRT was significantly higher in men with PSM compared to men with NSM status (p < 0.001), e.g., 28.9% (95CI: 26.6–31.3) and 12.9% (95CI: 11.7–14.0) after 10 years, respectively (). In multivariate modeling, PSM increased the risk of sRT with HR = 1.85 (95CI: 1.56–2.20, ). As preoperative PSA was frequently missing, risk of sRT was analyzed without PSA in the model as comparison, and the predictive value of the model did not change in a clinical meaningful extend (Supplementary Table S1). As most patients received sRT within 5 years of RP, we analyzed risk prediction modeling at year 5. Model validation showed that margin status at RP only had a minor, but statistically significant impact of the predictive ability of the model with AUC of 78.2% without margin status and 80.7% with margin status (p < 0.001), respectively (). Brier score decreased from 10.3% to 10.2% after addition of margin status to the model (p = 0.05). Both the reclassification diagram and the calibration plot showed marginally increased benefit when adding margin status to the model (data not shown).
Figure 2. Cumulative incidence of salvage radiation (sRT) and subsequent androgen deprivation therapy (ADT) after curatively intended radical prostatectomy (RP). (A) sRT incidence of the whole cohort, (B) subsequent ADT incidence of the whole cohort, (C) sRT incidence of the whole cohort stratified for margin status, (D) subsequent ADT incidence of the whole cohort stratified for margin status.
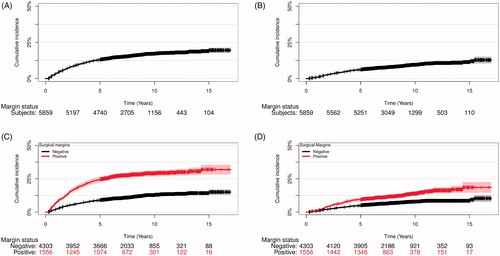
Figure 3. Receiver operating characteristic (ROC) curves showing predictive value of different models for predicting occurrence of salvage radiation therapy (sRT) after curatively intended radical prostatectomy (RP) at (A) 5, (B) 10, (C) 15 years. Abbreviations: Ncat: Lymph node status; AUC: Area under the curve; PSA: Prostate-specific antigen; Tcat: Pathological tumor category; Margin: Margin status.
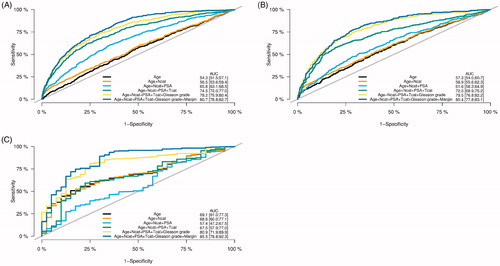
Table 2. Multivariate competing risk analysis of receiving salvage radiation therapy (sRT) and subsequent androgen deprivation therapy (ADT).
The overall incidence of sRT in men with pT2 tumors was 7.5% (95CI: 6.7–8.3), 10.7% (95CI: 9.6–11.7), and 12.7% (95CI: 11.0–14.45) after 5, 10 and 15 years, respectively. When stratified by margin status, incidence of sRT was 21.5% (95CI: 18.7–24.4) and 7.8% (95%CI: 6.8–8.9) at 10 years for men with PSM and NSM, respectively (Supplementary Figure S1(A)). In multivariate analysis, PSM was associated with a three-fold increased risk of sRT compared to men with NSM (HR = 2.93; 95%CI: 2.28–3.78, p < 0.001), Supplementary Table S2). Among pT2 tumors, margin status significantly increased AUC from 71.6% to 78.0% if included in the model (p < 0.001, Supplementary Figure S1(B)) and the Brier score decreased significantly from 7.1% to 6.9% after addition of margin status (p = 0.008) in 5-year prediction modeling.
In men with pT3 tumors the incidence of sRT was 27.9% (95CI: 25.7–30.1), 34.3% (95CI: 31.8–36.9), and 35.5% (95CI: 32.6–38.4) after 5, 10 and 15 years respectively. In multivariate analysis, PSM had a significant, but lower impact on risk of sRT compared to pT2 tumors (HR 1.37, 95%CI: 1.09–1.72, p = 0.006), Supplementary Table S3. In 5-year prediction modeling of sRT for pT3 patients, adding margin status to the model did not significantly change the AUC of 67.9% compared to 69.1% with addition of margin status (p = 0.08, Supplementary Figure S1(D)). Brier score did not change (19.3% with NSM status vs 19.1% with PSM) after addition of margin status to the model (p = 0.1).
To explore a possible effect of local treatment policies we investigated the incidence of sRT on an institutional level. The cumulative incidence of sRT at 10 years varied significantly among institutions with cumulative incidence of 19.6% (95CI: 17.5–21.8), 19.9% (95CI: 17.6–22.2), 14.2% (95CI: 12.3–16.2), 13.6% (95CI: 11.0–16.2), and 15.3% (95CI: 12.3–18.3), for hospital 1 to 5, respectively. In multivariate analysis using our own institution as reference we found significant increased risk of sRT at hospital 1, HR = 1.96 (95%CI: 1.56–2.48, p < 0.001) and at hospital 2, HR = 1.29 (95%CI: 1.02–1.64, p = 0.04, Supplementary Table S4(A)). When separately analyzing the effect of margin status for hospital 1 and hospital 3, a PSM was associated with increased risk of receiving sRT, HR = 2.26 (95%CI: 1.66–3.07, p < 0.001) at hospital 1 but not at hospital 3, HR = 1.14 (95% CI: 0.78–1.67, p = 0.51, Supplementary Table S5(A,B)). To further explore the impact of margin status on sRT between institutions, we performed a prediction model for a man of age 70, with pT2, N0 tumor at RP, preoperative PSA of 10 and specimen GS of 3 + 4. Comparing hospital 3 with hospital 1, we found that a PSM had no impact on sRT at hospital 3, whereas PSM doubled the risk of sRT after 10 years at hospital 1 (Supplementary Figure 2(A,B)).
Androgen deprivation therapy
The overall incidence of ADT following RP was 6.2% (95CI: 5.5–6.8), 9.4% (95CI: 8.6–10.3), and 11.3% (95CI: 10.0–12.7) after 5, 10, and 15 years, respectively. When stratified by margin status, the incidence of ADT was significantly higher in men with PSM compared to NSM (p < 0.001), e.g., 13.7% (95CI: 11.8–15.7) and 7.8% (95CI: 6.8–8.7) after 10 years, respectively (). In multivariate analysis, PSM was associated with an increased risk of receiving ADT compared to NSM (HR:1.39, 95CI: 1.09–1.76, p = 0.007, Supplementary Table 2(A)). Removing PSA from the model did not change the result in a clinical meaningful extend (Supplementary Table S1). As most patients received ADT within 10 years of RP we analyzed risk prediction modeling and validation at year 10 which showed that margin status at RP had no significant impact on the predictive ability of the model (AUC of 83.2% without margin status and 83.1% with margin status (p = 1), respectively). Brier score was unchanged after addition of margin status to the model (p = 0.50).
For pT2 patients the 5-, 10-, and 15-year incidence of ADT was 3.1% (95CI: 2.5–3.6), 5.3% (95CI: 4.5–6.1), and 6.8% (95CI: 5.6–7.9), respectively. For pT3 patients the corresponding figures were 14.4% (95CI: 12.7–16.1), 20.4% (95CI: 18.2–22.7) and 24.7% (95CI: 19.6–28.4), respectively. When stratified by pathological stage, PSM significantly increased the cumulative incidence of ADT (pT2, HR:1.32, 95CI: 0.87–2.00, p = 0.20, and pT3, HR:1.50, 95CI: 1.12–2.01, p = 0.007). AUC at 10 years for margin status did not significantly change in pT3 patients; 72.2 without margin status to 71.9 with margin status (p = 0.8). Brier score was also not significantly changed (p = 0.21).
In multivariate analysis, we found that hospital 4 (HR:0.60, 95CI: 0.37–0.97, p = 0.04) and hospital 5 (HR:0.36, 95CI: 0.18–0.73, p = 0.004) had a significantly lower risk of ADT (Supplementary Table 4). The impact of margin status and institution on subsequent ADT at 10 years was predicted for a man of age 70, with pT3a, N0 tumor at RP, preoperative PSA of 10 and specimen GS of 4 + 3 for hospital 3 and 5. The model showed that PSM regardless of hospital increased risk of receiving ADT (Supplementary Figure 2(C,D)).
Discussion
The purpose of this study was to address the use of sRT and ADT during long-term follow-up, to analyze how pathological factors affect the risk of subsequent therapies, and to assess how these factors differs between institutions. For many urologists, margin status after RP is translated to the technical quality of the procedure, although several studies point to the fact that tumor-, anatomical- and patient-characteristics play a large role for the risk of attaining a PSM after RP [Citation21]. Although both surgeon and patient may worry about the risk of BR, a PSM after RP is not synonymous with BR during follow-up. The risk of BR in men with PSM is modulated by several factors such as location, Gleason score at the margin, and length of the margin [Citation8,Citation22]. Furthermore, BR is only a surrogate marker for disease severity as many patients do not need further treatment despite rising PSA after RP [Citation23,Citation24].
The indication for sRT likely depends on several factors beyond the tumor characteristics, such as comorbidity, age, urinary function after RP, and local guidelines. Overall, this study demonstrated that one in five patients received sRT following RP. The rate of sRT is in concordance with the reported 29% use of sRT following RP shown by Cary et al. [Citation25]. Our study points out that PSM increased the risk of sRT following RP, especially in men with pT2 tumors. However, validation analysis demonstrated that adding margin status to the prediction model for sRT on top of the other characteristics only had modest effect on the AUC. Most importantly, our study demonstrates that PSM interplays with treating institution as predictive value of the margin status varied significantly between institutions, even in a stratified competing risk prediction modeling for a patient at high risk for undergoing sRT (Supplementary Figure S2(a + b)). This implies that, despite national guidelines, local tradition and other unknown factors have a large impact on decision making for sRT.
The strength of this study is related to the size and homogeneity of the cohort, national scale, and validity of the information included in the analysis originating from comprehensive national public databases. Consistent national guidelines throughout the study period and centralized treatment to highly specialized institutions reduce the risk of selection bias in terms of treatment choice. Yet, several factors can affect the generalizability of the results as the patients included originate from a virtually non-screened population and consequently, the patients could have more advanced cancers. The overall incidence of PSM was 27%, which could be interpreted as high; however, the patient population may not be comparable to contemporary reports [Citation26]. Furthermore, surgery was mainly performed by open retropubic approach, the surgical technique may, however, be of less importance as PSM-rates between open and robotic surgery have not been proven different in randomized trials [Citation27]. The incidence of PSM varied between institutions with a maximum 15% difference in the percentage of patients with PSM () unexplainable by patient characteristics. Although volume and surgical experience could play a role, we believe that other aspects, such as specimen handling, interpretation and definition of PSM could be part of the explanation [Citation5,Citation28,Citation29]. This is supported by a recent meta-analysis on pT2 PCa specimens, where Tan et al. demonstrated that cancer-specific factors accounted for only 15% of the variations in PSM rates, whereas other factors such as facility could explain nearly 25% of the variation [Citation30].
An overall increased risk of subsequent ADT in patients with a PSM was found. Intriguingly, the risk of receiving ADT was only significantly increased in pT3 patients and margin status did not significantly alter the model’s predictability for receiving ADT. Also, it was demonstrated that the predicted risk of commencing subsequent ADT was higher at hospital 3, but that increased risk of ADT due to PSM did not markedly differ per institution. The difference found raises concerns about the defining criteria and subsequent management of distant failure and initiation of subsequent ADT between hospitals.
A major limitation of the study is lack of preoperative PSA in a large proportion of men due to laboratorial policies of erasing historical data. However, analyses of the entire population including the ones without information on preoperative PSA showed that PSA did not affect HR for PSM significantly, indicating that factors beyond pre-operative PSA had the major influence on the risk of PSM. Furthermore, BR was not included as endpoint as PSA level at time of salvage therapy was unavailable, not allowing us to compare that endpoint to other previous studies. However, according to national guidelines, salvage therapies are not initiated prior to BR, and men receiving adjuvant therapy within 3 months from surgery have been excluded to avoid inclusion of patients participating in different clinical trials. It would have been very valuable to have known the PSA before sRT and ADT, as it would increase the understanding of the underlying decision-making between institutions. Unfortunately, these data were not available. The data in also show apparent differences in pathological factors between treating institutions and it is a concern if these differences explain the findings found in our analysis. Confounding factors and selection bias always limit the interpretation of retrospective analyses, although the statistical analysis, to a large extend, compensate for these concerns. Another limitation is the potential multicollinearity between the factors included in the multivariate analyses. To address this the variance inflation factor was calculated, which showed no major multicollinearity and considering the size of the cohort there should be no major influence (Supplementary Table S6). However, the conclusions of predictive value of each variable are therefore not overstated. A further limitation is that we do not know the background for the decision on subsequent therapy, and an interaction between the patient’s personal choice and the recommendation by the treating physician clearly influences the results. This may explain some of the wide inter-institutional variations in risk found despite similar patient groups and the same guidelines for initiating salvage treatment.
Conclusion
This study has demonstrated that PSM is associated with the risk of salvage radiation for local failure and initiation of ADT. Yet, model validation showed that margin status only to a small extent affects the risk for sRT and ADT. Most importantly, our results underline that an unknown factor at each treating institution may play a major role for initiation of sRT and ADT. Caution should be made when extrapolating the risk of subsequent therapies after RP for a patient with PSM given the lack of uniformity for reporting PSM, lack of clear definition of PSM, the effect of local guidelines, patient preference, and comorbidity. Therefore, until margin status assessment is uniform it will remain unclear if a patient should be followed differently based on their margin status.
Author contributions
Hein Vincent Stroomberg: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review and editing
Anne Sofie Friberg: Conceptualization, Data curation, Funding acquisition, Visualization, Writing – review and editing
John Thomas Helgstrand: Data curation, Funding acquisition, Project administration, Supervision, Writing – review and editing
Klaus Brasso: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing
Martin Andreas Røder: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing
Supplemental Material
Download MS Word (32.4 KB)Supplemental Material
Download MS Word (653.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data sharing
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- Compérat E, Camparo P, Srigley J, et al. Prise en charge de la pièce de prostatectomie radicale. Résultats de la conférence de consensus de la Société internationale d’uropathologie (ISUP). Ann Pathol. 2013;33(3):155–161.
- Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170(6 Pt 1):2292–2295.
- Tosoian JJ, Chappidi M, Feng Z, et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int. 2017;119(5):676–683.
- Ahlering TE, Skarecky D, Lee D, et al. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170(5):1738–1741.
- Evans AJ, Henry PC, Van Der Kwast TH, et al. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol. 2008;32(10):1503–1512.
- Van Der Kwast TH, Collette L, Van Poppel H, et al. Impact of pathology review of stage and margin status of radical prostatectomy specimens (EORTC trial 22911). Virchows Arch. 2006;449(4):428–434.
- Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;179(5 Suppl):S47–S51.
- Eastham JA, Kuroiwa K, Ohori M, et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007;70(5):965–969.
- Stephenson AJ, Wood DP, Kattan MW, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182(4):1357–1363.
- Røder MA, Berg KD, Loft MD, et al. The CPC risk calculator: a new app to predict prostate-specific antigen recurrence during follow-up after radical prostatectomy. Eur Urol Focus. 2018;4(3):360–368.
- Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66(6):1245–1250.
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer – 29-year follow-up. N Engl J Med. 2018;379(24):2319–2329.
- Zhang L, Wu B, Zha Z, et al. Surgical margin status and its impact on prostate cancer prognosis after radical prostatectomy: a meta-analysis. World J Urol. 2018;36(11):1803–1815.
- Helgstrand JT, Klemann N, Røder MA, et al. Danish Prostate Cancer Registry - methodology and early results from a novel national database. Clin Epidemiol. 2016;8:351–360.
- Parker C, Clarke NW, Cook A, et al. Timing of radiotherapy (RT) after radical prostatectomy (RP): first results from the RADICALS RT randomised controlled trial (RCT) [NCT00541047]. Ann Oncol. 2019;30:v883–v884.
- DaProCa. Gaeldende kliniske retningslinjer. Dansk Urol Cancer Grup 2015. https://ducg.dk/daproca-prostatacancer/kliniske-retningslinjer/. [cited 2020 Sept 3].
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417–428.
- Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138.
- Cortese G, Gerds TA, Andersen PK. Comparing predictions among competing risks models with time-dependent covariates. Stat Med. 2013;32(18):3089–3101.
- Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50(11):1–23.
- Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23(4):651–663.
- Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012;62(3):382–404.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. J Am Med Assoc. 1999;281(17):1591.
- Amling CL, Bergstralh EJ, Blute ML, et al. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165(4):1146–1151.
- Cary KC, Paciorek A, Fuldeore MJ, et al. Temporal trends and predictors of salvage cancer treatment after failure following radical prostatectomy or radiation therapy: an analysis from the CaPSURE registry. Cancer. 2014;120(4):507–512.
- Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: A systematic review and contemporary update. Eur Urol. 2014;65(2):303–313.
- Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388(10049):1057–1066.
- Vickers A, Bianco F, Cronin A, et al. The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010;183(4):1360–1365.
- Vainer B, Toft BG, Olsen KE, et al. Handling of radical prostatectomy specimens: total or partial embedding? Histopathology. 2011;58(2):211–216.
- Tan WS, Krimphove MJ, Cole AP, et al. Variation in positive surgical margin status after radical prostatectomy for pT2 prostate cancer. Clin Genitourin Cancer. 2019;17(5):e1060–e1068.
