Abstract
Background
Similarities in outcome between grade 3 endometrioid cancer and non-endometrioid histologies have been reported by a number of studies. Other reports, however, stated a significantly better prognosis for G3 endometrioid compared to type II histology. In this population-based study, we compared the outcome and treatment approaches of high-grade endometrial cancer patients with FIGO stages I–III depending on their histology.
Material and methods
284 high-grade endometrial cancer patients diagnosed between 1998 and 2015 were retrospectively analyzed. Overall survival (OS), recurrence-free survival (RFS), and recurrence rates were compared depending on histology.
Results
Type I G3 patients had a statistically significant OS advantage over women suffering from type II carcinoma (HR 1.527, 95%-CI 1.024–2.276; p = 0.038) and carcinosarcoma (HR 2.106, 95%-CI 1.270–3.493; p = 0.004) in univariable and multivariable Cox-regression analysis. RFS in Type I G3 was significantly superior compared to patients with carcinosarcoma (HR 1.719, 95%-CI 1.018–2.901; p = 0.043) and not significantly superior to type II patients (HR 1.368, 95%-CI 0.920–2.036; p = 0.122). Cumulative recurrence rates were significantly higher in carcinosarcoma compared to type I G3 (HR 2.217, 95%-CI 1.096–4.485; p = 0.027) in univariable analysis, but not after risk adjustment (HR of 1.472, 95%-CI 0.654–3.311; p = 0.350).
Conclusion
The prognosis of patients with type I G3 endometrial cancer patients seems to be significantly superior to patients with type II cancer and particularly carcinosarcoma. Systematic LND seemed to be beneficial in all of the three subtypes. The benefit of adjuvant treatment methods may differ between histologies.
Introduction
Endometrial cancer incidence is rising worldwide over the last decades [Citation1–4]. While the incidence of type I endometrial cancer remained stable, the more aggressive type II histologies are on the rise [Citation1–4].
The subdivision of endometrial cancer histologies into type I and II has been suggested by Bokhman in 1983. Type I cancer represent predominantly estrogen-driven low grade endometrioid histologies arising from intraepithelial carcinoma. Type II cancer was defined as estrogen-independent non-endometrioid cancer. Historically, type II histology comprises serous, clear cell cancer and mixed cell adenocarcinoma [Citation4,Citation5].
Over the years, new evidence has led to adaptions of this system [Citation6]. Carcinosarcoma (CS), a rare histology with malignant epithelial and stromal cells is classified as an aggressive form of endometrial cancer. Similarities in outcome and molecular alterations between grade 3 endometrioid cancer and non-endometrioid histologies have been reported by a number studies [Citation7–11]. Other studies, however, propose a significantly better prognosis for G3 endometrioid compared to serous or clear cell histology [Citation12,Citation13]. Even within the group of typ0e II endometrial cancer outcome seems to differ. The PORTEC-3 trial evaluating the effect of adjuvant radiochemotherapy on high-risk endometrial cancer reported serous histology as an independent risk factor [Citation14].
In this population-based study, we compared the outcome and treatment approaches of 284 patients with FIGO stage I-III high grade endometrial cancer depending on their histology.
Material and methods
Patient data was treated in conformity with the Declaration of Helsinki and Bavarian Cancer registration law.
Database and cohort
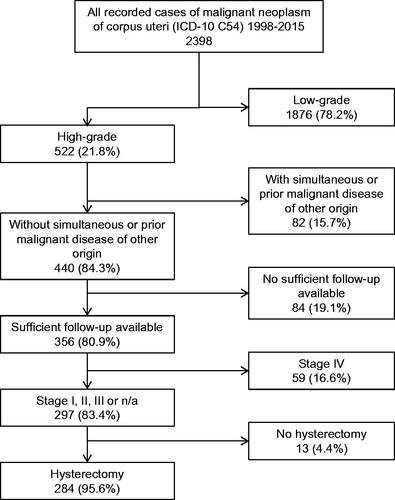
The Tumor Center Regensburg is a regional population-based clinical cancer registry with high-quality, cross-sectorial data on every patient diagnosed with cancer in the region of lower Bavaria and upper palatine with over 2.2 million inhabitants. Detailed information on the database has been described elsewhere [Citation15]. Patients with high-grade endometrial cancer diagnosed between January 1998 and December 2015 were identified (n = 522). Exclusion criteria were simultaneous or prior malignant disease of another origin, FIGO stage IV, inconclusive histology and insufficient documentation (≤ one medical record available). This led to a cohort of 284 patients ().
Definitions
Serous, clear cell and mixed cell carcinoma were subsumed under the term type II carcinoma. Grade 3 endometrioid, mucinous and adenosquamous were classified as type I G3 histology. Systematic lymphadenectomy was defined as 25 or more resected lymph nodes, elective lymphadenectomy as 1–24 removed lymph nodes. The definition was derived from the German guidelines valid from 2006 to 2018 suggesting a removal of at least 25 lymph nodes [Citation16,Citation17]. Pathology reports were reclassified according to the 2008 modification of the FIGO system.
Statistical analyses
Continuous data are expressed as median, minimum and maximum values. Categorical data is described using absolute frequencies and relative percentages. Overall Survival (OS), recurrence-free survival (RFS) and recurrence was estimated by means of Kaplan–Meier-method and Cox-regression model from the date of cancer diagnosis until the date of death of any cause, until the date of recurrence report, or last date recorded alive. All patients were classified as censored at a cutoff date 31 July 2020. Adjustments were made for potential confounding parameters age at diagnosis, Charlson Index score, year of diagnosis, tumor stage, extent of lymphadenectomy, salpingo-oophorectomy, residual local tumor mass (only relevant in OS) and lymphatic- or vascular invasion. Proportionality of hazards was evaluated for variables in multivariable COX-regression model by adding interaction terms with survival time and by plotting partial residuals of the estimated ratios against time. We did not apply Bonferroni or Bonferroni-Holm procedures in order to adjust for multiple testing. Statistical comparisons were made using a t-test for continuous data. Pearson’s Chi-square test was used for categorical variables. All t-tests were calculated two-sided. A p-value of 0.05 was defined as the threshold of statistical significance. Hazard ratios (HR) were considered significant if the corresponding Confidence Interval (CI) excluded 1. All calculations were done with the software packages SPSS 26 (Chicago, IBM).
Results
Patient and tumor characteristics
Two hundred and eighty-four patients with a mean age of 69.0 years (median 69.7, range 35.6–93.5) were included in the study. Mean follow-up was 10.0 years (median 9.1). The cohort comprises 67 (23.6%) patients with Type II carcinoma, 33 carcinosarcoma (11.6%), 184 Type I G3 (64.8%). Patients’ characteristics did not differ significantly between the histological subgroups in terms of patients’ age (p = 0.510), menopause status (p = 0.753), year of diagnosis (p = 0.755), FIGO stage (p = 0.604), or the rate of lymphatic (p = 0.091) or vascular invasion (p = 0.562, Table S1 and S2).
Treatment strategies
All patients underwent hysterectomy. In most patients (85.9%) the resection margins did not show signs of macroscopic or microscopic residual tumor (R0). Microscopic (R1) or macroscopic residual disease (R2) and unclear resection status (Rx) were present in 3.9% and 10.2% of patients, respectively. There was no significant difference in residual status between the histological groups (p = 0.942). Surgical lymph node staging was applied in 70.8%. There was no statistically significant difference in the frequency of lymphadenectomy in general (p = 0.660), nor in the regions it comprised between the histological subgroups (p = 0.506, Table S2).
A significant difference was observed for adjuvant treatment (p = 0.019). The rate of patients receiving radiochemotherapy was highest in carcinosarcoma patients (15.2%) compared to 5.4% and 11.9%% in type I G3 and type II patients, respectively. In contrast, the rate of radiotherapy without chemotherapy was lowest in carcinosarcoma (27.3%) compared to type I (58.7%) and type II (50.7%) patients.
Outcome
Overall survival (OS)
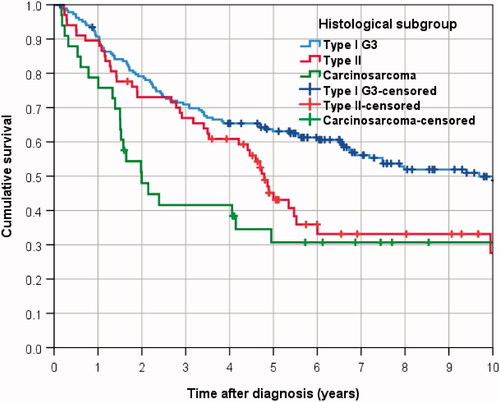
In the complete cohort, 166 patients (41.5%) deceased within the follow-up time (mean 10.0 years, median 9.1 years), yielding a 5-year OS rate of 55.4% and a median survival time of 6.6 years (). While 63.1% of type I G3 patients survived 5 years, the rate of patients alive 5 years from diagnosis was 45.1% for type II and 30.7% for carcinosarcoma ().
Table 1. Results from uni- and multivariable Cox-regression analyses for overall survival by histological type and other risk factors.
In Kaplan–Meier analysis type I G3 patients had a statistically significant survival advantage over women suffering from type II carcinoma (Log rank p = 0.018) and carcinosarcoma (p = 0.001). No significant difference was observed between type II and carcinosarcoma patients (p = 0.150).
The hazard ratio HR for OS derived from univariable Cox-regression analysis was 1.525 for type II patients (95%-CI 1.058–2.198; p = 0.024) and 2.178 for carcinosarcoma patients (95%-CI 1.378–3.442; p = 0.001), when using the type I cohort as reference (). The estimates for HR did not change substantially, when adjusting for confounding factors, rendering an HR of 1.527 for type II patients (95%-CI 1.024–2.276; p = 0.038) and 2.106 for carcinosarcoma patients (95%-CI 1.270–3.493; p = 0.004).
In multivariable analysis all factors considered for adjustment proved to be significant independent risk factors on OS except comorbidity and local residual tumor ().
Cumulative recurrence rate
Only patients known to be R0 resected were included in the analysis (n = 244). In the complete cohort, 63 patients had a recurrence during the follow-up, yielding a cumulative recurrence rate of 23.3% after 3 years, and of 28.3% after 5 years (Table S3).
Eight patients experienced a combined metastatic and loco-regional recurrence. The recurrence was only metastatic in 32 patients, only loco-regional in 16 cases and not further defined in 7 cases.
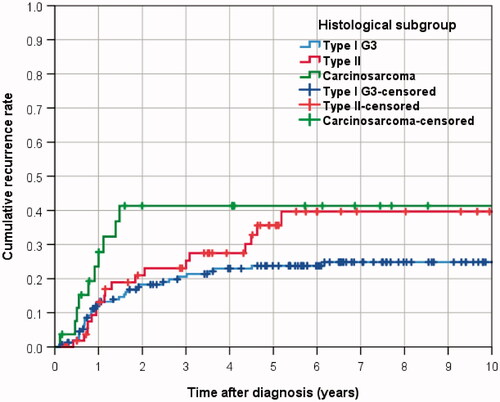
Cumulative recurrence rates were highest in carcinosarcoma with 41.3% after 3 years, showing a very early onset and reaching a plateau after 1.5 years (). Three-year recurrence rates for Type II and Type I G3 were 23.1% and 19.8%, respectively. After 5 years cumulative recurrence rates reached 41.3% for carcinosarcoma, 35.6% for Type II, and 23.8% for Type I G3. Differences were significant for carcinosarcoma compared to type I G3 (HR 2.217, 95%-CI 1.096–4.485; p = 0.027), but not for type II carcinoma (HR 1.504, 95%-CI 0.851–2.658; p = 0.160, , Table S3). The differences were not confirmed after risk adjustment in multivariable analysis, showing a HR of 1.472 (95%-CI 0.654–3.311; p = 0.350) for carcinosarcoma compared to type I patients.
Figure 3. Cumulative recurrence rates in endometrial cancer patients according to histologic subtype.
There was no significant difference in locoregional recurrence in multivariable or univariable analysis between the subtypes. 3- and 5- year locoregional relapses were both 16.4% for carcinosarcoma (Figure S1), 9.7% and 12.0% for type II patients and both 9.3% for type I cancer, respectively.
The highest cumulative rate of recurrences of distant metastases was observed in patients with type II carcinoma (3 years: 17.6%, 5 years: 31.1%), followed by carcinosarcoma (3 and 5 years both: 21.2%), and type I carcinoma (3 years: 11.0%, 5 years: 14.3%, Figure S1).
The rate of distant metastases relapse was significantly increased in the type II group (HR: 2.015, 95%-CI 1.123–3.969; p = 0.043) compared to the type I cohort in univariable analysis not in multivariable analysis (HR 1.997, 95%-CI 0.972–4.103; p = 0.060).
Recurrence-free survival (RFS)
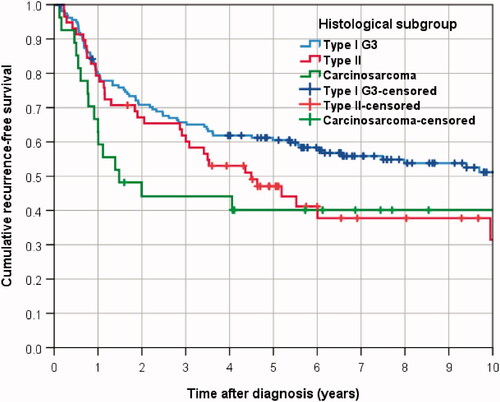
In the cohort of 244 R0-resected patients, 138 deceased or suffered from recurrence. After 5 years 55.1% of the patients were alive and showed no recurrence, the median time to death or relapse was 7.5 years, as estimated by the Kaplan–Meier method (Table S3).
The 5-year RFS was 60.5% for type I G3 patients, median RFS time was 10.9 years (). RFS was significantly superior to 5-year RFS for patients with carcinosarcoma (40.1%, median time 1.5 years (HR for carcinosarcoma 1.719, 95%-CI 1.018–2.901; p = 0.043) and not significantly superior to type II patients (47.0%, median time 4.5 years, HR for type II carcinoma 1.368, 95%-CI 0.920–2.036; p = 0.122). This result was confirmed in multivariable analysis, showing for carcinosarcoma vs type I patients a two-fold risk with an HR of 2.009 (95%-CI 1.106–3.647; p = 0.022), and a HR of 1.402 for type II vs type I patients (95%-CI 0.912–2.156; p = 0.124, Table S3).
Treatment effects
Adjuvant therapy in type I G 3 tumors
In 184 type I G3 patients 5-year OS was 70.0% for radiochemotherapy (RCT, n = 23), 71.2% for radiotherapy (RT, n = 151), 80.0% for chemotherapy (CTX, n = 11) and 45.9% for surgery only (SO, n = 99, Figure S2, Table S4). OS was significantly improved by RT (HR: 0.655, CI: 0.435–0.986, p = 0.042), not by RCT (HR: 0.551, CI: 0.169–1.796; p = 0.323) or CTX (HR: 0.465, CI: 0.112–1.929; p = 0.291), when compared to surgery only. Results were confirmed by multivariable analysis, showing a HR of 0.609 for RT vs SO (95%-CI 0.388–0.956; p = 0.031).
Of 159 type I patients with pathologically complete resection 35 patients recurred. Cumulative 5-year recurrence rates were 12.5% for RCT, 22.1% for RT, 40.0% for CT and 26.8% for SO. Adjuvant treatment with RT was significantly correlated with the reduction of recurrence rates in this cohort (HR 0.414, 95%-CI 0.197–0.872; p = 0.020) in multivariable analysis.
5-year RFS was 87.5% for RCT, 69.4% for RT, 60.0% for CTX and 39.1% for SO. RT appeared significantly superior to no adjuvant treatment (HR 0.535, 95%-CI 0.343–0.832; p = 0.006), which was still evident after risk adjustment in multivariable analysis (HR 0.577, 95%-CI 0.350–0.952; p = 0.031). There was no significant improvement in RFS for RCT compared to no adjuvant treatment in univariable analysis or multivariable analysis (Table S4).
Lymphadenectomy in type I G 3 tumors
In patients with type I G3 carcinoma 5-year OS was 73.6% for systematic LND (n = 77), 59.9% for elective LND (n = 40), 85.7% for unclassified LND (n = 14) and 44.4% for patients without LND (n = 53, Figure S3, Table S5). OS was significantly improved by systematic (HR: 0.434, CI: 0.228–0.826, p = 0.011) and unclassified LND (HR: 0.383, CI: 0.153–0.963, p = 0.041), not by elective LND (HR: 0.628, CI: 0.330–1.194, p = 0.156), when compared to no LND in multivariable analysis.
Cumulative 5-year recurrence rates were 18.6% for systematic LND, 33.8% for elective LND, 12.5% for unclassified LND and 27.3% for no LND. There was no significant difference in recurrence rates between the groups in univariable or multivariable analysis (Table S5).
5-year RFS was 74.6% for systematic LND, 57.0% for elective LND, 87.5% for unclassified LND and 35.2% for no LND. In univariable Kaplan–Meier-analysis elective LND (p = 0.047) and systematic LND (p = 0.001) yielded a significantly improved RFS compared to no LND. This effect was confirmed in multivariable analysis for systematic LND (HR 0.307, 95%-CI 0.146–0.664; p = 0.002), not for elective LND (HR 0.573, 95%-CI 0.280–1.174; p = 0.128).
Adjuvant therapy in type II tumors
In patients with type II carcinoma 5-year OS was 71.4% for radiochemotherapy (n = 8), 44.4% for radiotherapy (n = 34), 66.7% for chemotherapy (n = 3) and 34.1% for surgery only (n = 22, Figure S4, Table S6). Radiochemotherapy seemed to be superior to the other treatment options in Kaplan–Meier analysis, which proved not to be significant, when compared to surgery alone in univariable analysis (HR 0.252, 95%-CI 0.058–1.098; p = 0.066) but was significant in multivariable analysis (HR 0.072, 95%-CI 0.013–0.415; p = 0.003).
Five-year recurrence rates were 14.3% for radiochemotherapy, 47.0% for radiotherapy, 33.3% for chemotherapy and 26.1% for surgery only. No significant differences could be observed between the therapy groups in univariable (p = 0.293) or multivariable analysis (p = 0.433).
Five-year RFS was 85.7% for RCT, 39.6% for RT, 66.7% for CTX and 41.2% for SO. In Kaplan–Meier analysis, RCT was slightly superior to RT (p = 0.047) and SO (p = 0.049), which could not be confirmed in multivariable analysis (p = 0.087).
Lymphadenectomy in type II tumors
In patients with type II carcinoma 5-year OS was 61.0% for systematic LND (n = 32), 22.2% for elective LND (n = 12), 0% for unclassified LND (n = 2) and 33.1% for patients without LND (n = 21, Figure S5, Table S7). OS was significantly improved by systematic LND (p = 0.011) in univariable analysis, but not in multivariable analysis compared to no LND.
Cumulative 5-year recurrence rates were 28.3% for systematic LND, 41.7% for elective LND, 0% for unclassified LND and 58.0% for no LND. Recurrence rates were not significantly different between the groups in univariable or multivariable analysis (Table S7).
Five-year RFS was 63.2% for systematic LND, 33.3% for elective LND, 0% for unclassified LND and 23.1% for no LND. In univariable Kaplan–Meier-analysis systematic LND (p = 0.001) yielded a significantly improved RFS compared to no LND. This effect was confirmed in multivariable analysis for systematic LND (HR 0.230, 95%-CI 0.080–0.661; p = 0.006).
Discussion
This study retrospectively evaluated the OS, RFS and recurrence rates in a population of high-grade endometrial cancer patients. The OS of patients with type I G3 endometrial cancer was significantly higher compared to carcinosarcoma and type II cancer. Recurrence rates were highest in carcinosarcoma patients with a significant difference to type I G3 patients. RFS was superior in type I G 3 patients compared to carcinosarcoma. The difference in 5-year RFS of 60.5% versus 47.0% between type IG3 and type II cancer was not significant.
Several studies comparing the outcome between type I and type II endometrial cancer have been undertaken thus far. While a better prognosis for type I tumors in general are undisputed, comparisons between Type I G3 and type II endometrial cancer reach different conclusions.
Reynaers et al. studied a cohort of 123 early-stage endometrial cancer patients derived from a Dutch comprehensive cancer center. In that cohort, no difference in recurrence rates or disease-related mortality was reported with a 5-year RFS of roughly 70% for both endometrioid and non-endometrioid histologies [Citation9]. Ayeni et al. published a retrospective study on 370 endometrial cancer patients including FIGO stages I–IV. 119 patients had grade 3 endometrioid type, 211 had papillary serous type, and 40 had clear cell carcinomas. Overall survival was similar among different subtypes and did not differ in stage-for-stage comparative analyses [Citation7].
A National Cancer Database (NCDB) analysis, on the other hand, reported a slightly superior survival for type I G3 endometrial cancer compared to type II tumors [Citation12]. The study included 46.298 serous, clear cell or grade 3 endometrioid carcinoma. Similar results were obtained by a SEER-database analysis on 4180 women with high-grade endometrial carcinoma. OS as well as RFS of serous and clear cell carcinoma patients was lower compared to endometrioid G3 tumors [Citation18].
Even though the number of patients in our study was considerably lower than the studies conducted from databases mentioned above, we observed similar results. While OS was significantly poorer in the type II group, RFS and recurrence rates though nominally pointing toward poorer outcomes for type II cancer were not significant. The limited number of patients in this study might disguise the poorer outcome of type II cancers in terms of recurrence or RFS compared to type I G3. This could explain the discrepancy between our findings and the results from the SEER database in terms of RFS. Neither the SEER- nor the NCDB- study report on recurrence rates and can therefore not be compared with our study in this endpoint.
Even though it has become clear that carcinosarcoma represents a subtype of endometrial cancer rather than sarcoma, few studies have been undertaken to compare its prognosis to other forms of high-grade endometrial cancer. Amant et al. reported a worse prognosis of carcinosarcoma compared to other high-grade histologies in a cohort comprised of 137 high-risk patients stages I-IV [Citation19]. Felix et al, on the other hand, found no difference between carcinosarcoma patients and clear cell, serous or high-grade endometrioid cancer patients in terms of RFS and DSS [Citation20]. In our cohort, we observed the lowest rates of OS and RFS and the highest recurrence rates in carcinosarcoma patients which were statistically significant compared to type I G3, not to type II tumors. All recurrences took place rather within 3 years from diagnosis. This might have implications on the schedule of aftercare and suggests that carcinosarcoma represents a subtype with a particularly poor prognosis and early recurrences.
Explanations for the different results obtained by the aforementioned studies may lie in low patient numbers and potential difficulties regarding the grading of endometrioid cancers. Similar outcomes between high-grade endometrioid and type II histologies were predominantly reported by studies including few patients, while the database studies with high patient numbers observed poorer survival rates for type II tumors [Citation7,Citation9,Citation12,Citation20]. Another pitfall in these comparisons arises from the interobserver variability on pathological grading of endometrioid endometrial cancer with agreement rates of approximately 70% [Citation21]. In studies that include a higher rate of patients with tumors that could also be classified as grade 2, the prognosis of high-grade endometrioid cancers will most likely be better. Furthermore, ours was the only study that took comorbidities into account. As type II cancers tend to arise at higher age, comorbidities may play a role in disguising differences in outcome.
Recently, the cancer genome atlas project identified four novel prognostic molecular groups that may direct adjuvant therapy decisions in the future. So far, results from 6 studies- both from prospective and retrospective patient cohorts- have reported favorable results for POLE mutated patients. This prognosis does not seem to be affected by clinicopathological factors. The prognosis of p53 mutated, p54 wt and MSI patients is worse and, on the other hand, is worsened by unfavorable clinicopathological factors. Patients with p53 mutation present with the worst outcome. Unfortunately, our study lacks information on molecular subtypes. In the future, molecular classification should be obtained for all patients to further guide the decision process [Citation22].
Concerning LND and adjuvant treatment, we made some observations that need to be interpreted with a fair amount of reserve due to limited patient numbers and the retrospective design of the study. Nevertheless, systematic LND seemed to be beneficial in type IG3 and type II tumors. Whether this is due to the therapeutic effect of LND in high-risk tumors or rather due to an upstaging of occult stage III tumors is currently addressed in randomized trials [Citation23]. The benefit of adjuvant treatment methods (radiotherapy, radiochemotherapy, chemotherapy or none) may differ between histologies. It seems that RT is beneficial in type I G3 patients, whereas RCT improves OS and RFS in patients with type II tumors. The reason for the observed OS benefit of RT in type I tumors is most likely related to a selection bias as randomized trials reported a benefit for RFS and recurrence rates only. Furthermore, RCT improved RFS compared to RT only in high-risk carcinoma [Citation24,Citation25]. Due to the low number of carcinosarcoma patients, we did not report the effect of adjuvant therapy or LND in this subgroup.
The pattern of relapse seemed to differ in our cohort. We observed high rates of metastatic relapse in type II cancer. Loco-regional relapses appeared to be more frequent in carcinosarcoma, though not statistically significant. Further investigation is necessary to confirm these findings as they may implicate the need of different adjuvant treatment strategies.
Conclusion
In conclusion, the prognosis of patients with type I G 3 endometrial cancer patients seems to be significantly superior to patients with type II cancer and particularly carcinosarcoma. Systematic LND seemed to be beneficial in all of the three subtypes. The benefit of adjuvant treatment methods (radiotherapy, radiochemotherapy, chemotherapy or none) may differ between histologies.
Ethics approval
Due to the analysis of data from a clinical cancer registry no ethics approval was necessary. Patient data was treated in conformity with the Declaration of Helsinki and Bavarian Cancer registration law.
Statistic validity
The authors confirm that the statistical methods used are valid for the respective questions addressed.
Consent to participate/for publication
not applicable, due to the analysis of data from a clinical cancer registry
Author contributions
S.S, A.S., M.K., O.K. and T.P. conceived of the presented idea. T.S. and K.K. collected the data. M.G. performed the computations. The manuscript was drafted by S.S. The manuscript was revised by O.O., A.I., A.S. and O.K. All authors discussed the results and contributed to the final manuscript.
Supplemental Material
Download MS Word (43.9 KB)Supplemental Material
Download MS Word (62.4 KB)Supplemental Material
Download MS Word (181.4 KB)Disclosure statement
The authors declare that they have no conflict of interest
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to preservation of privacy but are available from the corresponding author on reasonable request.
References
- Faber MT, Frederiksen K, Jensen A, et al. Time trends in the incidence of hysterectomy-corrected overall, type 1 and type 2 endometrial cancer in Denmark 1978–2014. Gynecol Oncol. 2017;146(2):359–367.
- Kim H-J, Kim T-J, Lee Y-Y, et al. A comparison of uterine papillary serous, clear cell carcinomas, and grade 3 endometrioid corpus cancers using 2009 FIGO staging system. J Gynecol Oncol. 2013;24(2):120–127.
- Moore K, Brewer MA. Endometrial cancer: is this a new disease? Am Soc Clin Oncol Educ Book. 2017;37:435–442.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2 Pt 1):383–397.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17.
- Suarez AA, Felix AS, Cohn DE. Bokhman redux: endometrial cancer "types" in the 21st century. Gynecol Oncol. 2017;144(2):243–249.
- Ayeni TA, Bakkum-Gamez JN, Mariani A, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013;129(3):478–485.
- Buhtoiarova TN, Brenner CA, Singh M. Endometrial carcinoma: role of current and emerging biomarkers in resolving persistent clinical dilemmas. Am J Clin Pathol. 2016;145(1):8–21.
- Reynaers EAEM, Ezendam NPM, Pijnenborg JMA. Comparable outcome between endometrioid and non-endometrioid tumors in patients with early-stage high-grade endometrial cancer. J Surg Oncol. 2015;111(6):790–794.
- Voss MA, Ganesan R, Ludeman L, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124(1):15–20.
- Alektiar KM, McKee A, Lin O, et al. Is there a difference in outcome between stage I–II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002;54(1):79–85.
- McGunigal M, Liu J, Kalir T, et al. survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a National Cancer Database Analysis. Int J Gynecol Cancer. 2017;27(1):85–92.
- Park JY, Nam J-H, Kim Y-T, et al. Poor prognosis of uterine serous carcinoma compared with grade 3 endometrioid carcinoma in early stage patients. Virchows Arch. 2013;462(3):289–296.
- Boer S. d, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295–309.
- Scharl S, Papathemelis T, Kronberger K, et al. Does post-operative radiochemotherapy improve survival in high-grade endometrial cancer patients? Results of a population-based cohort analysis of a cancer registry. Arch Gynecol Obstet. 2018;297(5):1245–1253.
- DGGG. Diagnostik und Therapie des Endometriumkarzinoms. [Internet] [cited 2020 Dec 13]. Available from: https://www.dggg.de/leitlinien-stellungnahmen/leitlinien/leitlinie/diagnostik-und-therapie-des-endometriumkarzinoms-239/.
- Kommission Uterus der Arbeitsgemeinschaft Gynäkologische Onkologie e.V. in der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe e.V. sowie in der Deutschen Krebsgesellschaft e. V. Interdisziplinäre S2k-Leitlinie für die Diagnostik und Therapie des Endometriumkarzinoms. 2010. [Internet] [cited 2020 Apr 18]. Available from: https://www.awmf.org/uploads/tx_szleitlinien/032-034l_S2k_Endometriumkarzinom_01.pdf.
- Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94(5):642–646.
- Amant F, Cadron I, Fuso L, et al. Endometrial carcinosarcomas have a different prognosis and pattern of spread compared to high-risk epithelial endometrial cancer. Gynecol Oncol. 2005;98(2):274–280.
- Felix AS, Stone RA, Bowser R, et al. Comparison of survival outcomes between patients with malignant mixed mullerian tumors and high-grade endometrioid, clear cell, and papillary serous endometrial cancers. Int J Gynecol Cancer. 2011;21:877–884.
- Scholten AN, Smit V, Beerman H, et al. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004;100(4):764–772.
- Raffone A, Travaglino A, Mascolo M, et al. TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol. 2019;155(2):374–383.
- Mould T, Brand A, Nijman H, et al. STATEC: A randomised trial of non-selective versus selective adjuvant therapy in high risk apparent stage 1 endometrial cancer. J Clin Oncol. 2018;36(15_suppl):TPS5615–TPS5615.
- Hogberg T, Signorelli M, Oliveira CF de, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer-results from two randomised studies. Eur J Cancer. 2010;46(13):2422–2431.
- Creutzberg CL, Nout RA, Lybeert MLM, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e631-8–e638.