Abstract
Background
Surgical resection of brain metastases (BM) improves overall survival (OS) in selected patients. Selecting those patients likely to benefit from surgery is challenging. The Graded Prognostic Assessment (GPA) and the diagnosis-specific Graded Prognostic Assessment (ds-GPA) were developed to predict survival in patients with BM, but not specifically to guide patient selection for surgery. Our aim was to evaluate the feasibility of preoperative GPA/ds-GPA scores and assess variables associated with OS.
Methods
We retrospectively reviewed first-time surgical resection of BM from solid tumors at a Norwegian regional referral center from 2011 to 2018.
Results
Of 590 patients, 51% were female and median age was 63 years. Median OS was 10.3 months and 74 patients (13%) died within three months after surgery. Preoperatively tumor origin was unknown in 20% of patients. A GPA score could be calculated for 92% of the patients preoperatively, but could not correctly predict survival. A ds-GPA score could be calculated for 46% of patients. Multivariable regression analysis revealed shorter OS in patients with higher age, worse functioning status, colorectal primary cancer compared to lung cancer, presence of extracranial metastases, and more than four BM. Patients with preoperative progressive extracranial disease or synchronous BM had shorter OS compared to patients with stable extracranial disease.
Conclusion
Ds-GPA could be calculated in less than half of patients preoperatively and GPA poorly identified patients which had minimal benefit of surgery. Including status of extracranial disease improve prognostication and therefore selection to surgery for brain metastases.
Introduction
Brain metastases (BM) are the most frequent malignant brain tumors in adults, developing in 10–30% of all patients with cancer. The incidence is increasing [Citation1–4]. This may be explained by improved imaging, more patients admitted to diagnostic procedures and improved systemic treatments resulting in more patients living with metastatic disease. Lung cancer, melanoma, breast cancer and colorectal cancer are the most frequent tumor of origin in patients with BM (2). Expected overall survival (OS) is generally short, less than 5 months after BM diagnosis, although varying greatly between the primary cancers (10 months in breast cancer vs. two months for pancreatic cancer) [Citation3]. The morbidity is high and many patients suffer from severe symptoms and psychological distress [Citation5–7].
Anticancer treatment options for BM include radiotherapy (stereotactic radiotherapy (SRT) and whole brain radiotherapy (WBRT)), systemic treatments and surgery, together with palliative and supportive care. WBRT has been a cornerstone in the management of BM, and early studies have shown improved overall survival [Citation8] and symptom relief [Citation9]. SRT was initiated in the 1980s, and is now often preferred over WBRT, due to its equal effectiveness [Citation10], less cognitive side effects [Citation11–13] and shorter treatment time. Chemotherapy has traditionally not been a major management option in patients with BM, due to poor intracranial effect. However, novel systemic therapeutic approaches for BM have been proposed in the last decade, including molecular targeted therapies and immunotherapy. These are increasingly used in patients with BM from malignant melanoma and lung cancer, with promising results on survival and intracranial control [Citation14–17].
Surgical resection of BM has been shown to improve OS compared to radiotherapy alone in a randomized controlled trial [Citation18], while also giving a rapid improvement of neurological symptoms [Citation19]. The Management of Brain Metastases Guidelines were published in 2011, based on current evidence [Citation20]. The guidelines recommend surgery for patients with a limited number of BM, where lesions are more readily available through a single craniotomy, larger lesions >3 cm or in acute hydrocephalus. Moreover, surgery and consequential biopsies are highly important in cases of diagnostic uncertainty. The guidelines do not discuss how to evaluate extracranial disease when addressing surgery specifically; however, they acknowledge the importance of the extent of the systemic cancer in general.
In 1997, Gaspar et al. introduced the recursive partitioning analysis (RPA) to assess prognosis in patients with BM [Citation21]. In 2008 Sperduto et al. improved prognostication with the Graded Prognostic Assessment (GPA), based on the variables age, performance status, number of BM and presence of extracranial metastases [Citation22]. Nieder et al. validated the GPA on surgically treated patients shortly after, however with a very small sample size, only 64 patients [Citation23]. In 2012, Sperduto et al. included primary cancer diagnosis in the Diagnosis-Specific Graded Prognostic Assessment (ds-GPA) to further differentiate prognostic groups [Citation24]. The ds-GPA was updated for lung cancer and melanoma in 2017, renal and colorectal cancer in 2018 and 2019 and breast cancer in 2020 [Citation25–30]. These updates include diagnosis-specific molecular tumor markers such as EGFR and ALK for adenocarcinoma of the lung, BRAF for melanoma and HER2, estrogen receptor and progesterone receptor in breast cancer. The ds-GPA and recent updates were not specifically based on patients selected for surgical resection of BM and it remains unknown whether they can be used to assess a patient’s prognosis prior to BM surgery and to select or exclude patients to surgery. Further, the lowest ds-GPA score possible (0) has an expected OS of more than three months for each primary diagnosis. Therefore ds-GPA is insufficient to identify which patients have a very short OS and would have less clinically relevant benefit from a surgical resection. The precursor GPA is more readily available than the ds-GPA due to its fewer variables, but the GPA is based on 15-20 years old trials and it is unknown whether the survival estimates are valid for patients considered for surgery.
Acceptance to surgery for BM should be based on the experienced neurosurgeon`s overall assessment. However, due to the complexity of this evaluation the use of prognostic tools may reduce unwanted variation and improve decision making [Citation31]. Thus, we need more real-world data to support the selection of patients for surgical resection of BM.
We present preoperative characteristics of all patients who underwent first-time surgery for BM from 2011 to 2018 at Oslo University Hospital, a large regional referral center. We evaluated the possibility of calculating ds-GPA and GPA score preoperatively and assessed available variables associated with OS. Further, we investigated whether a GPA score could identify patients with OS less than three months.
Materials and methods
Oslo University Hospital is the only regional referral center for neurosurgery in the South-Eastern Norway Health Region, part of a public single-payer healthcare system, with a population of 3 million; 55% of the Norwegian population. Patients were identified through the Brain Tumor Registry at the Department of Neurosurgery. The historic cohort includes all patients ≥18 years who underwent surgical resection of one or more BM from a solid tumor in the time period 2011–2018.
We developed a predefined checklist for data extraction by round table discussion between in-house neurosurgeons and oncologists and reviews of the literature [Citation5,Citation21,Citation24,Citation32]. The variables include sex, age at surgery, comorbidity (ad modum Charlson [Citation33]), primary tumor, extracranial disease status, number of BM, Eastern Cooperative Oncology Group (ECOG) performance status, date of BM surgery and date of death. All variables were based on information available preoperatively.
Variables were extracted or estimated from patient charts and interpretation was done by the first author and discussed with members of the study group as necessary. Preoperative extracranial disease status was categorized as (1) stable: no documented new metastases or growing primary tumor in any imaging modality within the last three months prior to BM surgery, (2) progressive: growing primary tumor/metastases or new metastases three months prior to BM surgery, (3) synchronous: primary tumor discovered within one month prior to BM surgery/BM as first sign of disease or as (4) unknown disease status: known primary cancer, but no radiological staging three months prior to BM surgery. A clinically relevant survival from surgery was liberally set to be at least three months; comparable to what has been suggested for WBRT [Citation13,Citation34].
Ethics
The Norwegian Directorate of Health deemed this study a quality improvement project and issued a waiver of consent. The study was approved by the Data Protection Officer at Oslo University Hospital. All patients alive during the data collection were contacted and given the right to decline use of their data. Data storage and handling is done in accordance with the GDPR.
Statistical analyses
OS was estimated using the Kaplan-Meier estimator and the log rank test was used to assess differences in OS. Patients alive at the time of data analysis (27.07.2020) were censored for survival analyses. Hazard ratios were estimated by Cox’ proportional hazards model. The proportionality assumption was checked by visual inspection of log-log plots. With n = 590 patients included and median survival less than 1 year, the estimated power to detect HR ≥ 1.3 is at least 90% at a 5% significance level. All statistical analyses were performed in SPSS Statistics 25 (IBM Corp. Armonk, NY, USA).
Results
Incidence and patient characteristics
A total of 590 individual adults were included in the study, giving an incidence rate of craniotomy for BM of 2.5/100.000 per year. The median age of the study population was 63 years at the time of surgery, ranging from 18 to 89 years. 51% were female. The most common primary tumors were lung (33%), melanoma (16%), breast (9%) and colon (9%). 48% had comorbidities, 81% had an ECOG status of 2 or better and 54% had 0 or 1. A single BM was found in 64% while only 7% had more than four. Extracranial disease was considered stable in 29% of the patients and progressive in 18%, while synchronous disease was identified in 36% of the patients ().
Table 1. Preoperative patient characteristics.
Survival
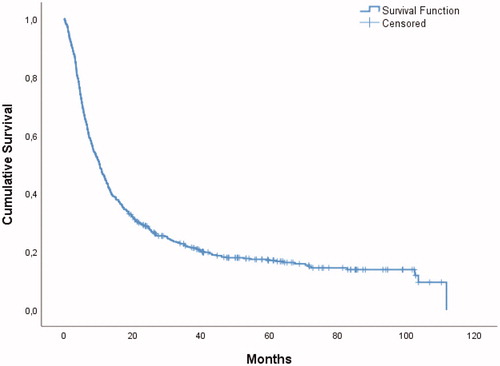
Median OS was 10.3 (95% CI: 8.8–11.7) months for the entire study population (). No patients were lost to follow-up. There was no significant trend in OS for the entire cohort over the course of the study period. Seventy-four patients (13%) died within three months after BM surgery.
Ds-GPA and GPA score availability
A ds-GPA score could be calculated in 274 patients (46%), as variables required for calculations were not available preoperatively (). The most frequent reasons for a lack of such a score were patients with synchronous disease and no histology from of primary tumor, and patients without radiological evaluation of extracranial disease within three months prior to BM surgery. A GPA score could be calculated in 540 (92%) patients. We found that GPA could differentiate prognostic groups, but the estimated OS was well below the confidence interval for all GPA scores, expect for GPA 3.5–4, due to a large confidence interval (). In logistic regression analysis GPA showed no ability to correctly predict survival less than three months.
Table 2 . GPA score and overall survival in months.
Preoperative factors and association with overall survival
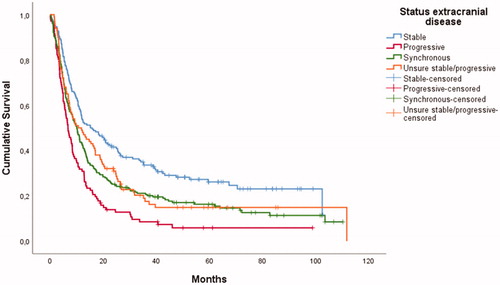
In unadjusted regression analyses the following variables were associated with shorter OS: male gender, increasing age, high ECOG status, colorectal primary tumor (compared to lung cancer), presence of comorbidity, extracranial metastases present, progressive, synchronous or unknown extracranial disease, (compared to stable extracranial disease), increasing number of BM and neurological deficits on examination. Breast cancer was associated with longer OS than lung cancer ().
Table 3. Cox regression analysis of preoperative characteristics.
In multivariable regression analysis we found an association with shorter OS in patients with age ≥70 (HR: 1.69 compared to <60), ECOG >2 (HR: 2.38 using ECOG=0 as the reference), colorectal primary tumor (HR: 1.56 using lung cancer as the reference), extracranial metastases (HR: 1.40), progressive extracranial disease (HR: 1.59) or synchronous disease (HR: 1.61) compared to stable extracranial disease (), more than four BM (HR: 1.93 using single BM as reference) and previous chemotherapy (HR: 1.50). The reduction in OS in patients with 2–4 BM compared to patients with single BM was small (HR: 1.22) and not formally statistically significant. Gender, comorbidity and neurological deficits on examination were not significantly associated with OS in the adjusted model. Breast cancer was associated with longer survival than lung cancer (HR: 0.55). OS was similar for patients with and without acute hydrocephalus ().
Discussion
We found that all variables in GPA were associated with OS, consistent with similar studies [Citation35–37]. The longer observed OS in our cohort could be that patients selected to surgery are generally healthier, as well as improvements in surgical techniques and systemic anticancer treatment the last 15-20 years. A GPA score was available in most patients and scores corresponded with OS. However, GPA could not correctly predict OS and was not suited for evaluation of short OS (less than three months), which limits the clinical use of GPA when patients are referred to surgery. However, patients with very short OS could be outliers that are almost impossible to identify preoperatively. On the other hand, ds-GPA could only be calculated in 46% of the patients based on preoperatively available variables, partly due to a high number of patients with synchronous disease and no histology of the primary tumor, and partly due to missing radiological evaluation of extracranial disease within three months prior to BM surgery in several patients. The lack of available variables could be explained by the often acute setting when patients are referred to surgery for BM. Biopsy and histopathological evaluation of primary cancer takes time and planning and may delay surgery. It is, however, possible to use imputing strategies to fill in missing data to estimate crude survival in clinical practice. This method would be less precise, but may still be useful. Ds-GPA score and association with OS in our cohort were not analyzed in primary tumor subgroups due to too small samples in renal, breast and colorectal cancer. Still, the lack of availability demonstrates the limited practical clinical use of ds-GPA when patients are evaluated for surgery for BM.
Median OS was 10.3 months, compared to 9.2 months in the previous report from our catchment area. Although the confidence intervals overlap, it falls well within the increasing data demonstrating improved OS for these patients over time [Citation38]. In 2019, Kavouridis et al. found a median OS of 15.4 months in a cohort of 1015 surgically treated BM patients [Citation35]. However, 63% of the patients had no extracranial metastases compared to 45% in our study; in addition to a larger number of patients with breast cancer (14 vs. 9%) who have longer expected OS. As seen in , more than 20% of patients with stable extracranial disease were still alive after 100 months, a stark contrast to the usually grim aspects of BM. It still proves difficult to predict more accurately who these patients are preoperatively: almost 10% of patients with progressive extracranial disease were also alive after 100 months ()
In the full survival model we included 98% of the patients for analysis. The lack of an Unknown option in several steps of the ds-GPA often makes it inadequate for many patients who are referred to surgery for BM. Further, we demonstrate the importance of status of extracranial disease and its association with OS; in that extracranial tumor growth three months prior to BM surgery indicates active and aggressive extracranial disease and a severe disease trajectory. In RPA, status of extracranial disease was included, categorized as controlled vs. uncontrolled primary tumor. This variable was removed in the GPA, which use the dichotomized presence of extracranial disease. We define progressive extracranial disease as radiologically verifiable tumor growth of primary tumor or extracranial metastases within three months prior to BM surgery, thus giving an objective evaluation of status of extracranial disease. Radiological examination of status of extracranial disease in addition to a GPA score would therefore improve prognostication and patient selection to surgery. However, this may be less relevant in patients with uncertain histopathological diagnosis, where surgery plays an important diagnostic role.
The incidence rate (2.5/100.00) of first time craniotomy for brain metastases in our cohort is consistent with the findings by Rogne et al. in the same demographic region in 2012 (2.6/100.000) [Citation38]. Median age, gender and composition of primary tumors were comparable to the findings of similar European studies [Citation38,Citation39], although international studies report a lower incidence of melanoma in their data [Citation35,Citation37,Citation40]. This finding is coherent with the high incidence of melanoma in the Nordic countries [Citation41]. We found an incidence of synchronous disease at 36%, similar to other studies that include this variable [Citation5,Citation40].
An important strength of this study is the use of a large unselected population, with regards to health care access. There are few recent studies including larger populations and these do not define extracranial disease other than present or not [Citation35]. Still, our cohort consists of patients already accepted for surgery and does not include all patients referred for BM surgery. There could be a decision bias over time, but we have not seen major changes in rate of craniotomy or changes in primary cancers during the study period. We are currently undertaking a prospective study on patients with BM, including patients rejected for surgery. Further, the study was too small to analyze subgroups of primary tumor mutation status, when only including preoperatively available information. The retrospective nature of the study may be considered a limitation and our primary outcome OS does not fully cover possible benefits to the patients. Even though 13% had an OS less than three months, they might still have benefited from surgery through relief of symptoms and improved functioning status. A recent study actually showed improved functioning status after surgical resection of BM in 33% of patients with OS less than three months [Citation40]. A prospective cohort study with patient reported outcome measures could further explore this important subject.
Conclusion
In this real-world cohort, a ds-GPA score was possible to calculate in less than half of patients at the time of referral for surgery for BM. GPA is more frequently available, but modern survival data vastly outperforms the GPA survival estimates. Prognostication is improved by considering the radiologically defined status of extracranial disease the last three months prior to BM surgery. Systematic radiological examinations could aid prognostication by including an objective evaluation of status of extracranial disease, and thereby improve future selection to surgery for BM.
Author contributions
Conception or design of the work: RRW, EOVM, OEY, SK, ES, NA, EH, MJH
Data collection: RRW
Data analysis and interpretation: RRW, EOVM, OEY, SK, ES, MJH
Drafting the article: RRW, EOVM, OEY, SK, ES, NA, EH, MJH
Critical revision of the article: RRW, EOVM, OEY, SK, ES, NA, EH, MJH
Final approval of the version to be published: RRW, EOVM, OEY, SK, ES, NA, EH, MJH
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Davis FG, Dolecek TA, McCarthy BJ, et al. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171–1177.
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54.
- Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521.
- Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017;19(2):162–174.
- Nieder C, Spanne O, Mehta MP, et al. Presentation, patterns of care, and survival in patients with brain metastases. Cancer. 2011;117(11):2505–2512.
- Wong J, Hird A, Kirou-Mauro A, et al. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol. 2008;15(5):25–45.
- Herman MA, Tremont-Lukats I, Meyers CA, et al. Neurocognitive and functional assessment of patients with brain metastases: a pilot study. Am J Clin Oncol. 2003;26(3):273–279.
- Tsao MN. Brain metastases: advances over the decades. Ann Palliat Med. 2015;4(4):225–232.
- Chao J-H, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682–689.
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060.
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044.
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409.
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014.
- Lazaro T, Brastianos PK. Immunotherapy and targeted therapy in brain metastases: emerging options in precision medicine. CNS Oncol. 2017;6(2):139–151.
- Hendriks LEL, Bootsma G, Mourlanette J, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer. 2019;116:182–189.
- Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681.
- Schapira E, Hubbeling H, Yeap BY, et al. Improved overall survival and locoregional disease control with concurrent PD-1 pathway inhibitors and stereotactic radiosurgery for lung cancer patients with brain metastases. Int J Radiat Oncol Biol Phys. 2018;101(3):624–629.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
- Mut M. Surgical treatment of brain metastasis: a review. Clin Neurol Neurosurg. 2012;114(1):1–8.
- Bhangoo SS, Congress of Neurologic Surgeons (CNS), Linskey ME, Kalkanis SN. Evidence-based guidelines for the management of brain metastases. Neurosurg Clin N Am. 2011;22(1):97–104.
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751.
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514.
- Nieder C, Geinitz H, Molls M. Validation of the graded prognostic assessment index for surgically treated patients with brain metastases. Anticancer Res. 2008;28(5b):3015–3017.
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425.
- Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–3784.
- Sperduto PW, Deegan BJ, Li J, et al. Estimating survival for renal cell carcinoma patients with brain metastases: an update of the Renal Graded Prognostic Assessment tool. Neuro Oncol. 2018;20(12):1652–1660.
- Sperduto PW, Fang P, Li J, et al. Estimating survival in patients with gastrointestinal cancers and brain metastases: An update of the graded prognostic assessment for gastrointestinal cancers (GI-GPA). Clin Transl Radiat Oncol. 2019;18:39–45.
- Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99(4):812–816.
- Sperduto PW, Mesko S, Li J, et al. Beyond an updated Graded Prognostic Assessment (breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107(2):334–343.
- Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (lung-molGPA). JAMA Oncol. 2017;3(6):827–831.
- Pawloski PA, Brooks GA, Nielsen ME, et al. A systematic review of clinical decision support systems for clinical oncology practice. J Natl Compr Canc Netw. 2019;17(4):331–338.
- Antoni D, Clavier JB, Pop M, et al. Institutional, retrospective analysis of 777 patients with brain metastases: treatment outcomes and diagnosis-specific prognostic factors. Int J Radiat Oncol Biol Phys. 2013;86(4):630–637.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Suh JH, Kotecha R, Chao ST, et al. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299.
- Kavouridis VK, Harary M, Hulsbergen AFC, et al. Survival and prognostic factors in surgically treated brain metastases. J Neurooncol. 2019;143(2):359–367.
- Liu Z, Lei B, Zheng M, et al. Prognostic factors in patients treated with surgery for brain metastases: A single-center retrospective analysis of 125 patients. Int J Surg. 2017;44:204–209.
- Pojskic M, Bopp MHA, Schymalla M, et al. Retrospective study of 229 surgically treated patients with brain metastases: Prognostic factors, outcome and comparison of recursive partitioning analysis and diagnosis-specific graded prognostic assessment. Surg Neurol Int. 2017;8(1):259.
- Rogne SG, Ronning P, Helseth E, et al. Craniotomy for brain metastases: a consecutive series of 316 patients. Acta Neurol Scand. 2012;126(1):23–31.
- Schackert G, Lindner C, Petschke S, et al. Retrospective study of 127 surgically treated patients with multiple brain metastases: indication, prognostic factors, and outcome. Acta Neurochir. 2013;155(3):379–387.
- Picarelli H, Oliveira ML, Marta GN, et al. Mortality, morbidity, and prognostic factors in the surgical resection of brain metastases: a contemporary cohort study. J Neurol Surg A Cent Eur Neurosurg. 2020;81(4):279–289.
- Tryggvadóttir L, Gislum M, Hakulinen T, et al. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):665–672.