Abstract
Objective
To report the long-term clinical outcomes of low-risk (LR) and intermediate-risk (IR) prostate cancer patients treated with low-dose-rate brachytherapy (LDR-BT) and external beam radiation therapy (EBRT).
Patients and Methods
Men with biopsy-proven low- and intermediate-risk prostate cancer received EBRT and LDR-BT in an Asian academic center from 2000 to 2019 were reviewed. Kaplan–Meier survival analysis was performed to compare biochemical failure-free survival (bFFS) and overall survival (OS) between LDR and EBRT in the low- and intermediate-risk cohorts.
Results
642 patients (521 EBRT and 121 LDR-BT) with low- and intermediate-risk prostate cancer were included for analysis. In the intermediate-risk group, 5- and 10-year bFFS was 96%, 89% and 86%, 61% for LDR-BT and EBRT, respectively. LDR-BT was associated with a statistically significant improvement of bFFS in the intermediate-risk cohort (HR 2.7, p = 0.02). In the low-risk cohort, no difference of bFFS was found between LDR-BT and EBRT (HR 1.9, p = 0.08). Hormone therapy was more common in EBRT than LDR-BT for intermediate-risk group (71% versus 44%, p < 0.05). Prostate cancer-specific mortality was low in both EBRT (1%) and LDR-BT (2%) cohorts. No significant difference in OS was found between LDR-BT and EBRT in low- and intermediate-risk group (HR 2.1, p = 0.2 and HR = 1.7, p = 0.3).
Conclusion
In our retrospective study, LDR-BT is associated with superior bFFS compared with EBRT in Asian men with intermediate-risk prostate cancer.
Background
Low dose rate brachytherapy (LDR-BT) and external beam radiotherapy (EBRT) are two curative radiotherapy modes for men with localized prostate cancer. Adding androgen deprivation therapy (ADT) and increasing radiation dose are both effective strategies to improve clinical outcomes. Several phase 3 studies have demonstrated the superior biochemical control with dose-escalated EBRT [Citation1–3]. Recently, the adaption of moderate- and ultra-hypofractionation radiotherapy significantly reduces treatment duration and provides a more convenient EBRT option [Citation4,Citation5]. Comparison of long-term effectiveness among different modern radiation modalities in low- and intermediate-risk prostate warrants more compelling evidence.
Randomized data directly comparing the effectiveness of EBRT with brachytherapy are limited. Two studies comparing brachytherapy plus EBRT with EBRT alone by Sathya et al. and Hoskin et al. demonstrated better local and biochemical control [Citation6,Citation7]. However, EBRT dose in the standard arm of these studies was considered to be sub-optimal by the current standard dose of 74–81 Gy [Citation1–3]. Several retrospective studies have attempted to compare the effectiveness of different radiation modalities and demonstrated superior biochemical control of LDR-BT comparing with modern EBRT regimens in low- and intermediate-risk prostate cancer [Citation8,Citation9]. However, interpretation of retrospective studies can be challenging due to unbalanced baseline features like age, Gleason score, tumor stage, PSA and unequal use of hormonal therapy in different treatment groups [Citation10].
Our study aimed to report the long-term results of EBRT and LDR-BT for low- and intermediate-risk prostate cancer in an Asian population.
Material and methods
Patient data were extracted from a prospective database of the Department of Radiation Oncology, National Cancer Center, Singapore. All patients with biopsy-proven low- and intermediate-risk prostate cancer according to D’Amico Risk Classification treated in our institution from 2004 to 2019 were reviewed. Ethics approval (CIRB ref: 2016/2020/B) was obtained for the current study. All the patients were staged by clinical examination (DRE), pelvic imaging (CT or MRI), bone scan and PSA.
Radiotherapy techniques included image-guided intensity-modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) according to published protocols [Citation1,Citation4,Citation5]. LDR-BT patients were restricted to I125 permanent seed implantation to a minimum dose of 144 Gy. Short-term hormonal therapy (4–6 months) was used at the discretion of treating physicians. In the analysis, EBRT regimens included conventional fractionation (CFRT, 74–78 Gy in 1.8–2.0 Gy per Fraction), moderate hypofractionation (HFRT, 60 Gy in 3 Gy per fraction) or ultra-hypofractionation (UHRT, 36.3–37.5 Gy in 7.3–7.5 Gy per fraction) radiotherapy. All the patients were followed up by attending radiation oncologists and urologists according to institution protocol. Treatment-related toxicities were evaluated with RTOG/EORTC Radiation Toxicity Grading by radiation oncologists.
Outcomes
Outcome measures were biochemical Failure Free Survival (bFFS) and Overall Survival (OS). bFFS was defined as the time from initial diagnosis until biochemical recurrence defined by Phoenix criteria or death from all causes, surviving patients without disease recurrence were censored at their date of the last follow-up. OS was defined as the time from diagnosis to death from all causes, surviving patients were censored at their date of last follow-up.
Statistical methods
Survival curves were estimated using the Kaplan–Meier method. Differences in survival curves between LDR-BT and EBRT patients were assessed using the log-rank test. Follow-up time was estimated using the reverse Kaplan–Meier method. Univariable and multivariable analyses were performed to assess the association between bFFS with clinicopathologic and treatment characteristics. Variable selection for multivariable analysis was performed using backward elimination, by optimizing AIC and Harrel’s C-index. Statistical significance was defined by two-sided p-value less than 0.05. All statistical analyses were performed using R software (version 3.6.3).
Results
A total of 657 patients with low- and intermediate-risk prostate cancer who received radiotherapy were identified from a prospectively established database. 161 EBRT and 68 LDR-BT patients were eligible for low-risk group analysis; 360 EBRT and 53 LDR patients were included for the intermediate-risk comparison. The baseline characteristics in low- and intermediate-risk cohorts are shown in . The median follow-up was 7.4 (Interquartile Range, IQR, 4.0–9.7) years for the entire cohort and varied among different groups. In the low-risk cohort, LDR-BT patients had a median follow-up of 8.6 (IQR, 6.4–10.6) years, whereas EBRT patients had a median follow-up of 7.6 (IQR, 5.7–9.6) years. In the intermediate-risk cohort, median follow-up was 8.4 (IQR, 4.7–10.7) years and 6.9 (IQR, 2.7–9.4) years for LDR-BT and EBRT, respectively. Hormonal therapy was more commonly used for EBRT than LDR-BT (72% versus 44%, p < 0.05).
Table 1. Baseline characteristics for patients with low- and intermediate-risk prostate cancer.
In the low-risk cohort, 87% of patients were treated with CFRT (74 Gy), 10%, and 3% of patients received UHRT and HFRT radiotherapy, respectively. 5- and 10-year bFFS for () were 92% and 85% for LDR and 87% and 70% for EBRT, respectively. The 5- and 10-year OS estimates () were 96% and 91% for LDR, and 91% and 87% for EBRT, respectively. No significant difference was found for both bFFS (HR 1.9, 95% CI 0.9–4.0, p = 0.08) and OS (HR 2.1, 95% CI 0.7–6.2, p = 0.2) between LDR and EBRT.
Figure 1. Kaplan–Meier curves of biochemical failure-free survival (bFFS) (A,C) and overall survival (OS) (B,D) by risk group. External beam radiation therapy (EBRT) versus LDR brachytherapy (LDR-BT) for (A,B) low-risk patients receiving LDR-BT (n = 68) versus EBRT (n = 161); (C,D) intermediate-risk patients receiving LDR-BT (n = 53) versus EBRT (n = 360).
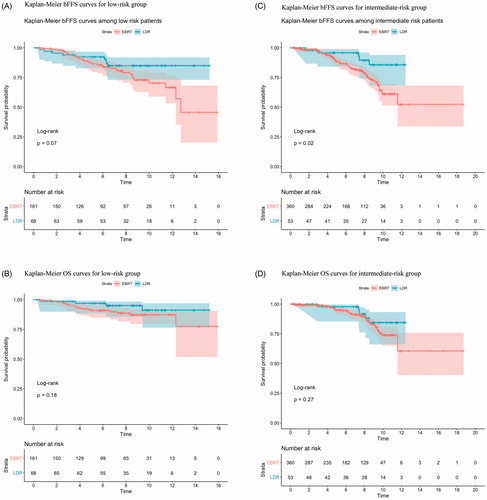
In the intermediate-risk cohort, CFRT (74 Gy in 37 fractions) was the most common regimen (78%), and 18% of patients received UHRT (36.3–37.5 Gy in 5 fractions). 5- and 10-year bFFS of EBRT are 89% and 61%, while 5- and 10-year bFFS of LDR are 96% and 86%. LDR was associated with statistically significant improvement in bFFS (HR 2.8, 95% CI 1.1–6.9, p = 0.02). In total, 2 of 53 (4%) LDR patients and 11% of EBRT patients experienced biochemical failure. No OS difference was found between LDR and EBRT (HR 1.7, 95% CI 0.7–4.2, p = 0.3).
Prognostic factors like tumor stage, Gleason Score, PSA, LDR-BT, and hormonal therapy are included in the multivariable analysis for bFFS in the intermediate-risk group. LDR-BT and hormonal therapy were associated with superior bFFS in multivariable analysis. Comparing to LDR-BT, EBRT was associated with inferior bFFS (HR 3.1, 95% CI 1.2–8.0, p = 0.017). Both hormonal therapy and radiotherapy were selected in the final multivariable Cox regression model and remained statistically significant in the reduced MV model ().
Table 2 . Univariate and multivariable analyses (full model and reduced model) of covariates associated with biochemical failure-free survival (bFFS) in the intermediate-risk group.
A total of 59 (11%) patients in EBRT cohort died during follow-up and prostate cancer-related death was 2% (8/535). In the LDR cohort, all-cause of death was 8% (10/122) and prostate cancer-specific death was 2% (3/122). Grade 2 and above genitourinary (GU) toxicity was 9% (11/122) in LDR cohort and 3% (18/535) in EBRT cohort. Grade 2 and above gastrointestinal (GI) toxicity was 3% (14/535) in EBRT group; no high grade (grade 2 and above) GI toxicities were found in the LDR cohort.
Discussion
Our retrospective study reported the long-term outcomes of Asian men with low- and intermediate-risk prostate cancer received LDR-BT and EBRT. Overall, prostate cancer-specific death was low in both cohorts, and all cause of death are similar between two treatment groups. These findings were consistent with PROTEC study, prostate cancer-specific mortality was low (0.4–1% at 10 year) in low- and intermediate-risk patients, and disease control and overall survival are similar between surgery and radiotherapy [Citation11]. Our results of biochemical control of LDR-BT and EBRT in the low-risk group were consistent with previous literature [Citation8,Citation9]. Goldner et al. reported their long-term outcomes of LDR and EBRT (74 Gy), and both treatments achieved comparable biochemical control results in low-risk prostate cancer [Citation12].
In intermediate- and high-risk prostate cancer, further dose escalation of EBRT improved biochemical control in several prospective studies [Citation1–3]. Brachytherapy alone or brachytherapy as a boost after EBRT provides the opportunity for further treatment intensification [Citation6,Citation7]. In ASCENDE-RT study, LDR-BT boost after EBRT provides superior biochemical controls for intermediate- and high-risk prostate cancer compared with EBRT of 78 Gy and hormonal therapy [Citation13]. Two Canadian studies performed match-pair analysis and LDR-BT was associated with superior bFFS compared with EBRT in intermediate-risk prostate cancer [Citation9].
Hypofractionation significantly reduces the total treatment number and holds important advantages in terms of patient convenience and resource utilization. Moderate hypofractionation has emerged as new alternative options for localized prostate cancer. Both CHHiP and PROFIT studies demonstrate similar long-term cancer control and toxicities comparing HFRT (60 Gy with 3 Gy per fraction) with CFRT (74 Gy or 78 Gy with 2 Gy per fraction) in localized prostate cancer [Citation4,Citation5,Citation14]. A recent meta-analysis of 7 studies and 6795 patients reported similar safety and efficacy UHRT compared with CFRT and HFRT [Citation15].
Brachytherapy delivers an ablative dose to the entire glands but also exposes a higher dose to normal tissue. Our results demonstrated a higher rate of GU toxicities in LDR-BT (9% versus 3%), especially urethra stricture required surgical intervention (3%). A previous study on LDR-BT also reported higher late GU toxicities and worse Health-Related Quality of Life (HR-QOL) in comparison with EBRT [Citation16]. It remains debatable whether better PSA control is necessary for all patients, especially no prostate cancer-specific survival benefit was associated with LDR-BT and patients experienced more GU toxicities.
Our study does have several limitations and needs interpretation within context. First, a retrospective study with a relatively small sample size only included patients with completed clinical data and potentially introduce biases during patient selection. Second, intermediate-risk prostate cancer is a heterogeneous group of diseases [Citation17]. Information on percent positive core, primary and secondary pattern of Gleason Score was incomplete for all patients and known prognostic clinical and pathological factors are not well-balanced in EBRT and LDR cohorts. Further, the most common EBRT dose (74 Gy) adopted in our study might be considered as sub-optimal by the current standard. The median follow-up duration (7.4 years) in our study is relatively short for low- and intermediate-risk patients and further observation is still required to demonstrate the long-term bFFS benefit of LDR-BT over EBRT.
Direct comparison of LDR and contemporary EBRT regimens, especially moderate- and ultra-hypofractionation, are needed for future studies. Last, the role of hormonal therapy with different radiotherapy regimens in intermediate-risk prostate cancer remains unclear, and hormonal therapy was at the discretion of the treating physician in our study.
Despite these limitations, our study added long-term data of the Asian population to the existing literature of EBRT and LDR-BT in low- and intermediate-risk prostate cancer. Different EBRT regimens also reflect the migration of practice patterns in our practice over the past two decades.
In conclusion, our retrospective study demonstrated that LDR-BT is associated with improved bFFS compared with EBRT in Asian men with intermediate-risk prostate cancer.
Supplemental Material
Download MS Word (31 KB)Disclosure statement
Melvin L.K. Chua reports personal fees from Astellas, Janssen, Bayer, Pfizer, MSD, personal fees and non-financial support from AstraZeneca, personal fees and grants from Ferring, personal fees and non-financial support from Varian, non-financial support from Decipher Biosciences, non-financial support from MedLever, and consults for immunoSCAPE Inc., outside the submitted work.
Additional information
Funding
References
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473.
- Heemsbergen WD, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110(1):104–109.
- Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063.
- Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open. 2019;2(2):e188006.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial [published correction appears in Lancet Oncol. 2016 Aug;17 (8): e321]. Lancet Oncol. 2016;17(8):1047–1060.
- Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23(6):1192–1199.
- Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103(2):217–222.
- Smith GD, Pickles T, Crook J, et al. Brachytherapy improves biochemical failure-free survival in low- and intermediate-risk prostate cancer compared with conventionally fractionated external beam radiation therapy: a propensity score matched analysis. Int J Radiat Oncol Biol Phys. 2015;91(3):505–516.
- Pickles T, Keyes M, Morris WJ. Brachytherapy or conformal external radiotherapy for prostate cancer: a single-institution matched-pair analysis. Int J Radiat Oncol Biol Phys. 2010;76(1):43–49.
- Suvarna VR. Real world evidence (RWE) – are we (RWE) ready? Perspect Clin Res. 2018;9(2):61–63.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424.
- Goldner G, Pötter R, Battermann JJ, et al. Comparison of seed brachytherapy or external beam radiotherapy (70 Gy or 74 Gy) in 919 low-risk prostate cancer patients. Strahlenther Onkol. 2012;188(4):305–310.
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395.
- Lehrer EJ, Kishan AU, Yu JB, et al. Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: a systematic review and meta-analysis of phase III randomized trials [published online ahead of print, 2020 Apr 28]. Radiother Oncol. 2020;148:235–242.
- Pinkawa M, Piroth MD, Fischedick K, et al. Self-assessed bowel toxicity after external beam radiotherapy for prostate cancer–predictive factors on irritative symptoms, incontinence and rectal bleeding. Radiat Oncol. 2009;4(1):36.
- Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64(6):895–902.
