Abstract
Background
The prognosis of pancreatic cancer is poor and new treatment strategies are urgently needed. To identify non-cancer drugs that could be re-purposed for cancer, we investigated the association between the use of selected drugs and cancer-specific mortality in a nationwide cohort of pancreatic cancer patients.
Material and methods
The study is based on linkage between the Cancer Registry of Norway and the Norwegian Prescription Database, comprising 2614 pancreatic cancer patients diagnosed between 2007 and 2014. We evaluated the association between use at diagnosis of a pre-defined list of non-cancer drugs, including metformin, antihypertensives, and statins, and pancreatic cancer-specific mortality, using Cox regression. Patients were defined as users of a particular drug if it was prescribed before diagnosis, and the prescription covered the date of diagnosis.
Results
In total, 2096 (80.2%) patients died from pancreatic cancer; median survival was 6 months. Statin users (n = 621) had lower mortality (hazard ratio (HR): 0.86; 95% confidence interval (CI) 0.76–0.97) compared to non-users (n = 1993). This association was more pronounced (P-heterogeneity 0.062) in users of hydrophilic (n = 37, HR: 0.61; 95% CI 0.42–0.90) than lipophilic (n = 587, HR: 0.87; 95% CI 0.78–0.98) statins. An indication for lower mortality (HR: 0.85; 95% CI 0.69–1.05) was observed in users of non-selective beta-blockers (n = 113) compared to non-users (n = 2501). Notably, when compared to users of other antihypertensives (n = 643), users of non-selective beta-blockers (n = 40) had lower mortality (HR 0.67; 95% CI 0.47–0.96). The use of other drugs, including selective beta-blockers and metformin, was not associated with mortality.
Conclusion
The findings suggest an association between the use of statins and non-selective beta-blockers and reduced pancreatic cancer mortality, and add to the literature supporting the design of randomised clinical trials to evaluate those drugs in the management of pancreatic cancer.
Background
Pancreatic cancer has a poor prognosis, with a 5-year survival rate of 5–9%, representing the 4th and 7th leading cause of cancer-related deaths in Europe and worldwide, respectively [Citation1–3]. There is currently no standard screening program for pancreatic cancer, and most patients remain asymptomatic until the disease reaches an advanced stage. Surgical resection, followed by adjuvant chemotherapy, is offered to the minority of patients with resectable disease and is still regarded as the only potentially curative treatment. Immunotherapy and targeted therapy are promising emerging therapeutic options, although no clinically relevant benefit in survival has been shown so far [Citation4]. New treatment strategies for this group of patients are therefore urgently needed.
Repurposing available and well-known non-cancer drugs is a recognised strategy that could provide new and affordable treatment options, with few safety concerns [Citation5]. Epidemiological studies have suggested that various non-cancer drugs, such as β-adrenergic receptor antagonists (beta-blockers) [Citation6,Citation7], angiotensin receptor blockers, angiotensin-converting enzyme (ACE) inhibitors [Citation8,Citation9], statins, and metformin [Citation10,Citation11], may be associated with improved pancreatic cancer prognosis. Most of these studies did not provide results for pancreatic cancer-specific mortality [Citation7,Citation8], which may misrepresent the significance of the findings since the use of these drugs is associated with serious comorbidities. In addition, dose-response analyses were also lacking in many of the studies [Citation7,Citation8,Citation11].
Some of the abovementioned drugs, including metformin [Citation12–14], angiotensin receptor blockers [Citation15], and statins [Citation16] have been evaluated in clinical trials. However, many of these trials have been negative or inconclusive. Therefore, the effectiveness of repurposed drugs remains uncertain, and new large epidemiological studies are needed to identify directions for further investigation and to support the initiation of new clinical trials.
Based on the existing evidence for drug repurposing for pancreatic cancer [Citation17], we studied the association between the use of 14 promising drugs/drug classes and pancreatic cancer prognosis in a nationwide cohort of pancreatic cancer patients. The list included proton-pump inhibitors, metformin, anticoagulants, antiplatelets, diuretics, beta-blockers, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, statins, non-aspirin non-steroid anti-inflammatories (NA-NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, bisphosphonates, and antidepressants.
Material and methods
Data source and study population
This study used data from a large linkage between Norwegian registries, which has been detailed elsewhere [Citation18]. Briefly, all subjects, aged 18–84, with a cancer diagnosis between 1st January 2007 and 31st December 2014 were identified from the Cancer Registry of Norway (CRN). For these patients, cancer information, sex and age were obtained from the CRN. Prescribed medications were collected from the Norwegian Prescription Database, classified according to the Anatomical Therapeutic Chemical (ATC) classification system [Citation19]. The Norwegian Patient Registry (NPR) provided comorbidity information (only available from 2008). This information was summarised using the Norwegian Patient Registry Index, which is a 15 level modified version of the Charlson Comorbidity Index. [Citation20]. Time and cause of death were obtained from the Norwegian Cause of Death Registry.
We identified all patients with a first primary pancreatic cancer diagnosis (n = 3599) using the International Classification of Diseases (ICD) 10th revision (code C25) and excluded patients with cancer of the pancreas other than adenocarcinoma (n = 422) and missing histology (n = 563), leaving us with a final study sample of 2614. Pancreatic cancer patients were followed up until death, emigration, or 31 December 2014, whichever occurred first.
The study was approved by The Regional Committee for Medical and Health Research Ethics in the South-East Health Region of Norway (2016/352 Identifikasjon av karsinogene og kjemopreventive effekter av reseptpliktige legemidler) and the Norwegian Data Protection Authority.
Drug use
We examined the use of the following drugs/drug classes: proton pump inhibitors, metformin, anticoagulants, antiplatelets, diuretics, beta-blockers, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, statins, NA-NSAIDs, COX-2 inhibitors, bisphosphonates, and antidepressants. Based on previous evidence supporting a differential effect between selective and non-selective beta-blockade [Citation21], beta-blockers were separated into selective and non-selective. Statins were separated into lipophilic and hydrophilic and antidepressants into non-selective monoamine reuptake inhibitors, selective serotonin reuptake inhibitors, and other antidepressants. The drugs were combined into classes because the number of users of specific drugs was not sufficient to perform drug-level analyses for drugs other than metformin. Combination drugs were categorised in all groups they belonged to, for instance, beta-blockers and thiazides (C07B) were categorised as both beta-blockers and diuretics. ATC codes for each drug class are reported in Supplementary Table S1. Patients were defined as users of a particular drug at diagnosis if they were prescribed that drug before diagnosis, and the prescription covered the day of diagnosis. We chose to define exposure as use at diagnosis to recapitulate the situation where treatment strategy is decided at diagnosis and commences soon after. The duration of a prescription was based on the defined daily dose (DDD), which is the assumed average maintenance dose per day for a drug used for its main indication in adults, defined by the WHO International Working Group for Drug Statistics Methodology. We assumed that the duration of each prescription equalled the prescribed DDD.
Statistical analysis
Cause-specific hazard ratios (HRs) with 95% confidence intervals (CIs) for pancreatic cancer-specific mortality were estimated with Cox regression, comparing users and non-users of a particular drug or drug class. Adjustments were made for sex, age as a continuous variable, comorbidity index (categorised as 0, 1–2, and 3–7) [Citation20], and stage (categorised as localised, regionally advanced, and metastatic). All drugs under investigation were also adjusted for each other, that is, the main drug groups were adjusted for the other main drug groups while the subgroups were adjusted for main drug groups and subgroups if available. Possible sex differences in associations between drug use and pancreatic cancer mortality were tested with interaction terms. Missing values of comorbidity index and stage were handled by multiple imputations with chained equations, assuming that the missing values are missing at random [Citation22]. The imputation model included the outcome and all drug exposures and adjustment variables. We imputed 25 data sets and the estimates and standard errors were combined using Rubin’s rule [Citation23]. Proportionality was assessed with Schoenfeld residuals.
Adjusted cumulative incidence functions for pancreatic cancer-specific mortality according to the use of beta-blockers and statins were estimated with flexible parametric models using splines with 5 degrees of freedom by averaging over the predicted individual level cumulative incidence functions [Citation24]. In these analyses missing values were handled with missing indicators.
We first analysed the association between use at diagnosis of the 14 pre-defined drugs/drug classes (including sub-groups of beta-blockers, statins, and antidepressants) and pancreatic cancer mortality in all patients, and also stratified by stage (i.e., localised or regionally advanced and metastatic). For metformin, beta-blockers, angiotensin receptor blockers, and statins, we further analysed the associations according to the low and high average daily dose. This was estimated by the sum of all DDDs in a two-year window prior to diagnosis (excluding the last prescription) divided by the number of days between first and last prescription. The average daily dose was categorised as low (≤1 DDD per day) and high (>1 DDD per day). In secondary analyses we compared users of beta-blockers and angiotensin receptor blockers with users of other antihypertensives (diuretics (C03) and/or calcium channel blockers (C08) and/or ACE inhibitors), and compared metformin users with users of any anti-diabetics (A10) not containing metformin. Patients using either (a) beta-blockers and other antihypertensive drugs, or (b) angiotensin receptor blockers and other anti-hypertensives, or (c) metformin and other anti-diabetic drugs, were not defined as users of either individual drug but were categorised in combination groups.
Heterogeneity between hydrophilic and lipophilic statins, between selective and non-selective beta-blockers, and between non-selective monoamine reuptake inhibitors and selective serotonin reuptake inhibitors were tested with an active comparator design. In those analyses, users of only hydrophilic statins were compared to users of only lipophilic statins, and users of only non-selective beta-blockers were compared to users of only selective beta-blockers, and users of only non-selective monoamine reuptake inhibitors were compared to users of only selective serotonin reuptake inhibitors. All tests were two-sided with a 5% statistical significance level. All statistical analyses were performed using R version 3.4.4 (http://cran.r-project.org) and the R-package mexhaz, version 1.5 for adjusted cumulative incidence curves.
Results
We analysed data from 2614 pancreatic cancer patients, with a median follow-up of 6 months. We observed 2255 (86.3%) deaths from any cause and 2096 (80.2%) from pancreatic cancer. The median age at diagnosis was 67 years, 51.4% of the patients were men and 62.9% had no registered comorbidity (). The majority of patients were diagnosed with metastatic disease (60.8%) and did not receive surgery with curative intent for the primary tumour (78.7%). Characteristics of the patients according to the use of the 14 drugs are reported in Supplementary Tables S2A-N.
Table 1. Baseline characteristics of 2614 patients diagnosed with pancreatic adenocarcinoma in Norway from 2007 to 2014.
Patients using proton pump inhibitors (HR:1.16; 95% CI 0.05–1.30), anticoagulants (HR: 1.32; 95% CI 1.16–1.50), antiplatelets (HR: 1.14; 95% CI 1.01–1.29), NA-NSAIDs (HR:1.29; 95% CI 1.12–1.49) or antidepressants (HR:1.32; 95% CI 1.12–1.56) had higher pancreatic cancer mortality compared to non-users ().
Table 2. Drug use at diagnosis and cancer-specific mortality.
Patients using statins (HR: 0.86; 95% CI 0.76–0.97) had lower pancreatic cancer mortality compared to non-users (1-year cumulative probability of pancreatic cancer-specific death 0.64 (95% CI 0.61–0.68) versus 0.67 (95% CI 0.65–0.69), ). We observed an indication for heterogeneity (p = 0.062) between hydrophilic statin users (HR: 0.61; 95% CI 0.42–0.90), 1 year cumulative probability 0.54 (95% CI 0.43–0.65) versus 0.67 (95% CI 0.66–0.69) and lipophilic statin users (HR: 0.87; 95% CI 0.78–0.98), 1 year cumulative probability 0.65 (95% CI 0.61–0.68) versus 0.68 (95% CI 0.66–0.70). The lower mortality in users of hydrophilic statin was more pronounced with high dose (HR:0.57; 95% CI 0.35–0.93) than the low dose (HR:0.76, 95% CI 0.42–1.39) (), although was not significantly different (p = 0.465). In lipophilic statin users, no clear dose-response trend was observed.
Figure 1. Cumulative incidence function for pancreatic cancer specific death for beta-blocker and statin use adjusted for sex, age, comorbidity index, stage and use of other drugs/drug classes under investigation.
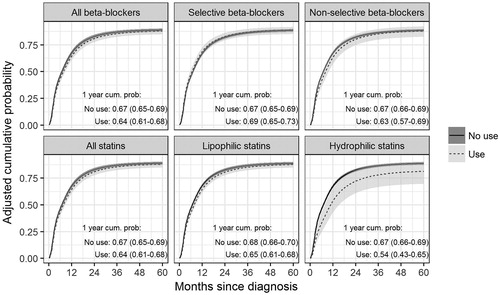
Table 3. Association between drug dose in the 2 years before diagnosis and cancer-specific mortality.
The use of beta-blockers was not associated with pancreatic cancer mortality. The HR for patients using non-selective beta-blockers compared to non-users was 0.85, 95% CI 0.69–1.05 () and for high dose non-selective beta-blocker users it was 0.74, 95% CI 0.54–1.01 (). When compared to patients using diuretics and/or calcium channel blockers and/or ACE inhibitors, non-selective beta-blocker users had a significantly lower mortality (HR: 0.67, 95% CI 0.47–0.96, ). Metastatic patients using angiotensin receptor blockers had lower pancreatic cancer mortality (HR: 0.84, 95% CI 0.72–0.99) compared to non-users (). No other drugs were associated with pancreatic cancer mortality. Results were similar in non-metastatic and metastatic pancreatic cancer patients, in patients resected and not resected for their primary tumour (data not shown), and no interactions with sex were observed. In an exploratory analysis, no significant heterogeneity between non-selective monoamine reuptake inhibitors and selective serotonin reuptake inhibitors was observed (p = 0.220).
Table 4. Use at diagnosis of selected drugs and cancer-specific mortality in comparison to other drugs used for the same indication.
Discussion
To evaluate the potential for repurposing existing drugs for pancreatic cancer, we investigated the association between the use of 14 non-cancer drugs and cancer-specific mortality in a nationwide cohort of 2,614 pancreatic cancer patients. We found a beneficial association between use of statins, especially hydrophilic statins, and the use of non-selective beta-blockers, and pancreatic cancer specific mortality. We found no association between use of metformin, diuretics, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers, COX-2 inhibitors or bisphosphonates, and pancreatic specific mortality.
Supporting the finding that statins may have beneficial effects, a meta-analysis by Jian-Yu et al., comprising six cohort studies published between 2012 and 2016, found that the use of statins among pancreatic cancer patients was associated with a 25% mortality reduction compared to no-use [Citation10]. The association was later confirmed in some epidemiological studies [Citation25–28], but not in others [Citation7,Citation29]. Statins have been shown to exert their anti-cancer effects via multiple mechanisms [Citation30]. The first is through the inhibition of the mevalonate/cholesterol synthesis pathway, which has been implicated in cancer cell proliferation, survival, motility, and invasion [Citation31] as well as in chemotherapy [Citation32] and radiation resistance [Citation33]. Additionally, statins have been shown to decrease the expression of inflammatory cytokines and to modulate the expression of a number of genes involved in angiogenesis and inflammation [Citation30].
We observed that the longer survival among statin users was more pronounced for users of hydrophilic statins, such as pravastatin than users of lipophilic statins, such as simvastatin. Because of the difference in their chemical structure, it is plausible that hydrophilic and lipophilic statins exert different anti-cancer effects [Citation34]. Although there are differences with regards to dosing, drug interactions, and adverse events, the indication for lipophilic and hydrophilic statins is the same, and the choice of type of statin is mainly based on the clinician’s preference [Citation35]. Due to this, it is unlikely that the observed survival difference between hydrophilic and lipophilic statins is only due to confounding by (contra-) indication.
Evaluation of the possible protective effect of hydrophilic versus lipophilic statins has generated conflicting results. The only clinical trial of statins in pancreatic cancer patients to date is a phase II study that reported no clinical benefit of adding low-dose simvastatin to gemcitabine for advanced pancreatic cancer patients [Citation16]. Only the hydrophilic rosuvastatin was found to improve pancreatic cancer survival in a meta-analysis comprising 4 epidemiological studies reporting separate estimates for simvastatin, pravastatin, lovastatin, atorvastatin, and rosuvastatin [Citation11]. Simvastatin has been shown to inhibit the growth of cancer cell lines more effectively than pravastatin in a dose-dependent manner [Citation36], and lipophilic statins but not hydrophilic statins were found to modulate the expression of genes involved in the mevalonate pathway [Citation37]. Additionally, mice with pancreatic tumours treated with a daily dose of the hydrophilic rosuvastatin that approximates the hypocholesterolemic dose used in humans, survive longer than mice treated with the same dose of the lipophilic atorvastatin, simvastatin, and fluvastatin [Citation38].
Pancreatic cancer cells express both β1- and β2-adrenergic receptors (AR) [Citation39]. Accumulating evidence suggests that sympathetic nervous system activation of the β-adrenergic receptor (βAR) signalling may play a role in the regulation of pancreatic cancer by driving tumour cell invasion, increasing cell proliferation, and inhibiting apoptosis [Citation40,Citation41] the epidemiological evidence for the effect of beta-blocker use on pancreatic cancer survival is scarce and conflicting, with studies reporting detrimental effects [Citation42], beneficial effects [Citation6,Citation7,Citation41] or no association [Citation43]. We found indications for longer survival among non-selective beta-blocker users when compared to users of other antihypertensives, although this was not observed when beta-blockers were considered as a single class. The effect of perioperative use of propranolol, a non-selective beta-blocker, is currently being investigated in ongoing clinical phase II trials of pancreatic cancer patients undergoing surgery (NCT03838029, DRKS00014054) in addition to the effect of propranolol combined with etodolac in metastatic pancreatic cancer patients [Citation44]. Only two epidemiological studies have stratified according to βAR selectivity, with conflicting results. One study reported a longer median overall survival in non-selective beta-blocker users than in selective beta-blocker users [Citation41], while the other study reported no significant difference between non-selective and selective beta-blocker users in terms of mortality reduction [Citation6].
Metformin has postulated antitumour effects via lowering insulin/insulin-like growth factor 1 levels and activation of AMP-activated protein kinase which inactivates the mTOR pathway [Citation45,Citation46]. Metformin use was found to be associated with improved survival in the meta-analysis by Jian-Yu et al., comprising 8 cohort studies and 2 clinical trials [Citation10]. However, two-phase II clinical trials in advanced/metastatic pancreatic cancer patients reported no improvement in 6 months overall and progression-free survival in the metformin arm compared to the placebo arm [Citation12,Citation13]. In an uncontrolled phase II trial with locally advanced or metastatic pancreatic cancer patients, only 32% of the patients had stable disease after 8 weeks and 40% of the patients experienced treatment-related toxicity [Citation14]. Our finding of no improved survival in metformin users, when compared to either non-users or users of other anti-diabetics, is in line with these trials.
We found higher pancreatic cancer mortality in patients using proton pump inhibitors, anticoagulants, antiplatelets, NA-NSAIDs, and antidepressants. Although these might be true associations, a plausible explanation is confounding by indication, in which the condition treated with the drug led to a worse prognosis, rather than the drug itself [Citation47]. For instance, both pre- and post-cancer depression have been suggested to increase cancer mortality [Citation48,Citation49], and this might explain the observed detrimental association in users of antidepressants. One of the most common symptoms of pancreatic cancer is abdominal pain [Citation50], which has been shown to be more prevalent in advanced disease [Citation51,Citation52]. It is therefore plausible to assume that the patients treated with NA-NSAIDs generally had more advanced disease at diagnosis, which could explain the higher mortality in NA-NSAID users. The higher pancreatic cancer mortality in users of anticoagulants may be partly explained by the fact that patients diagnosed with cancer during or after an episode of venous thromboembolism have been shown to be more likely to be diagnosed with advanced disease [Citation53]. It is also plausible that the use of these drugs may be associated with worse outcomes because the drugs interact negatively with cancer treatment. For instance, proton pump inhibitors reduce the absorption of tyrosine kinase inhibitors, used in the treatment of some metastatic pancreatic cancer patients, leading to lower survival in concomitant users [Citation54].
The primary strength of our study is the linkage of nationwide registries of high quality and completeness. As a result, the findings are not subject to selection bias or recall bias of drug use. Our definition of drug usage also prevented the occurrence of immortal-time bias, a common bias in pharmaco-epidemiology studies. However, our study also has limitations. First, there might be some overestimation of drug use as we do not know if the patients complied with their treatment, and some underestimation of drug use among the oldest patients as drugs given in institutions such as retirement homes are not included in the Norwegian Prescription Database. To the extent this occurred, it would lead to weaker associations by misclassifying some users as non-users and some non-users as users. Secondly, as we did not consider drug use after diagnosis, it is possible the observed associations may be weakened by patients who stopped taking a drug after diagnosis. However, the percentage of users who collected at least one prescription after diagnosis was high for the majority of drugs (Supplementary Table S3), suggesting that associations are not substantially weakened.
Third, we did not have information about radiation and chemotherapy that would have allowed us to test more precise hypotheses about the potential role of these non-cancer drugs in combination with the current treatment options for pancreatic cancer. Fourth, we lacked detailed information on prognostic factors, such as tumour size, lymph node involvement, and metastasis, which could have reduced some of the possible confoundings by indication for NA-NSAIDs and anticoagulants. Furthermore, smoking and obesity have been shown to decrease survival in pancreatic cancer patients [Citation55,Citation56]. It is, therefore, possible that the improvement in survival is underestimated among users of drugs with smoking and obesity-related indications, including cardiovascular drugs, statins, and metformin since we could not adjust for smoking and body mass index. Fifth, information about comorbidities was only available from 2008 onwards, and therefore was missing for patients diagnosed in 2007. However, since all estimates were adjusted for all 14 drugs/drug classes, the possible residual confounding by comorbidities should be minimal. Sixth, we did not have information about the daily dose used by individual patients but relied on the DDD, which is the assumed average maintenance dose per day. This has implications for the definition of use at diagnosis since the duration is calculated based on the DDD and not the individual daily dose. However, in a sensitivity analysis that assumed 0.5 DDDs per day (i.e., twice the duration of exposure) the results were similar (Supplementary Table S4), suggesting that the possible misclassification of some users as non-users does not substantially impact the findings. Finally, we chose to not adjust for multiple testing as the study was more explorative than confirmative in nature [Citation57].
In conclusion, in this nationwide cohort of pancreatic cancer patients, we found evidence of an association between the use of statins and non-selective beta-blockers and a reduction in pancreatic cancer mortality. Our findings support the design of randomised clinical trials that aim to test the real efficacy of those two drug classes in the management of pancreatic cancer patients.
Supplemental Material
Download MS Word (42.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349.
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387.
- Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348.
- Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology-patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12(12):732–742.
- Udumyan R, Montgomery S, Fang F, et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 2017;77(13):3700–3707.
- Beg MS, Gupta A, Sher D, et al. Impact of concurrent medication use on pancreatic cancer survival-SEER-medicare analysis. Am J Clin Oncol. 2018;41(8):766–771.
- Cerullo M, Gani F, Chen SY, et al. Impact of angiotensin receptor blocker use on overall survival among patients undergoing resection for pancreatic cancer. World J Surg. 2017;41(9):2361–2370.
- Mc Menamin ÚC, Murray LJ, Cantwell MM, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control. 2012; (2):221–230.
- Jian-Yu E, Graber JM, Lu SE, et al. Effect of metformin and statin use on survival in pancreatic cancer patients: a systematic literature review and meta-analysis. CMC. 2018;25(22):2595–2607.
- Tamburrino D, Crippa S, Partelli S, et al. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: a meta-analysis. Dig Liver Dis. 2020;52(4):392–399.
- Reni M, Dugnani E, Cereda S, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clin Cancer Res. 2016;22(5):1076–1085.
- Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–847.
- Braghiroli MI, de Celis Ferrari AC, Pfiffer TE, et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience. 2015;9:563.
- Nakai Y, Isayama H, Ijichi H, et al. A multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2. Invest New Drugs. 2013;31(5):1294–1299.
- Hong JY, Nam EM, Lee J, et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73(1):125–130.
- Pantziarka P, Verbaanderd C, Sukhatme V, et al. ReDO_DB: the repurposing drugs in oncology database. Ecancermedicalscience. 2018;12:886.
- Andreassen BK, Støer NC, Martinsen JI, et al. Identification of potential carcinogenic and chemopreventive effects of prescription drugs: a protocol for a Norwegian registry-based study. BMJ Open. 2019;9(4):e028504.
- ATC Structure and principles [Internet]. WHO Collaborating Centre for Drug Statistics Methodology. Geneva (Switzerland): WHO; 2018 [cited 2020 April 5]. Available from: https://www.whocc.no/atc/structure_and_principles/
- Nilssen Y, Strand TE, Wiik R, et al. Utilizing national patient-register data to control for comorbidity in prognostic studies. Clin Epidemiol. 2014;6:395–404.
- Yap A, Lopez-Olivo MA, Dubowitz J, et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth. 2018;121(1):45–57.
- Bartlett JW, Seaman SR, White IR, et al. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res. 2015;24(4):462–487.
- Rubin D. Multiple imputation for nonresponse in surveys. New York (NY): John Wiley and Sons; 2004.
- Kipourou DK, Charvat H, Rachet B, et al. Estimation of the adjusted cause-specific cumulative probability using flexible regression models for the cause-specific hazards. Stat Med. 2019;38(20):3896–3910.
- Huang BZ, Chang JI, Li E, et al. Influence of statins and cholesterol on mortality among patients with pancreatic cancer. JNCI J Natl Cancer Inst. 2017;109(5):djw275.
- Hamada T, Khalaf N, Yuan C, et al. Prediagnosis use of statins associates with increased survival times of patients with pancreatic cancer. Clin Gastroenterol Hepatol. 2018;16(8):1300–1306.
- Abdel-Rahman O. Statin treatment and outcomes of metastatic pancreatic cancer: a pooled analysis of two phase III studies. Clin Transl Oncol. 2019;21(6):810–816.
- Moon do C, Lee HS, Lee YI, et al. Concomitant statin use has a favorable effect on gemcitabine-erlotinib combination chemotherapy for advanced pancreatic cancer. Yonsei Med J. 2016;57(5):1124–1130.
- El-Refai SM, Brown JD, Arnold SM, et al. Epidemiologic analysis along the mevalonate pathway reveals improved cancer survival in patients who receive statins alone and in combination with bisphosphonates. JCO Clin Cancer Inform. 2017;1:1–12.
- Gong J, Sachdev E, Robbins LA, et al. Statins and pancreatic cancer. Oncol Lett. 2017;13(3):1035–1040.
- Mullen PJ, Yu R, Longo J, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16(11):718–731.
- Guillaumond F, Bidaut G, Ouaissi M, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112(8):2473–2478.
- Souchek JJ, Baine MJ, Lin C, et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br J Cancer. 2014;111(6):1139–1149.
- Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10(11):625–642.
- Kheloussi S. Considerations in the approach to approprate statin selection. US Pharm. 2018;43(7):22–26.
- Menter DG, Ramsauer VP, Harirforoosh S, et al. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS One. 2011;6(12):e28813.
- Gbelcová H, Rimpelová S, Ruml T, et al. Variability in statin-induced changes in gene expression profiles of pancreatic cancer. Sci Rep. 2017;7:44219.
- Gbelcová H, Lenícek M, Zelenka J, et al. Differences in antitumor effects of various statins on human pancreatic cancer. Int J Cancer. 2008;122(6):1214–1221.
- Weddle DL, Tithoff P, Williams M, et al. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22(3):473–479.
- Kim-Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–47.
- Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75–90.
- Shah SM, Carey IM, Owen CG, et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72(1):157–161.
- Springate DA, Ashcroft DM, Kontopantelis E, et al. Can analyses of electronic patient records be independently and externally validated? Study 2–the effect of β-adrenoceptor blocker therapy on cancer survival: a retrospective cohort study. BMJ Open. 2015;5(4):e007299.
- Bhattacharyya G, Babu K, Bondarde S, et al. Effect of coadministered beta blocker and COX-2 inhibitor to patients with pancreatic cancer prior to receiving albumin-bound (Nab) paclitaxel (abstract) 2015. J Clin Oncol. 2015;33(3):302.
- Amin S, Boffetta P, Lucas AL. The role of common pharmaceutical agents on the prevention and treatment of pancreatic cancer. Gut Liver. 2016;10(5):665–671.
- Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–156.
- Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818–1819.
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11):1797–1810.
- Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. The effect of depression on stage at diagnosis, treatment, and survival in pancreatic adenocarcinoma. Surgery. 2012;152(3):403–413.
- Schmidt-Hansen M, Berendse S, Hamilton W. Symptoms of pancreatic cancer in primary care: a systematic review. Pancreas. 2016;45(6):814–818.
- Bakkevold KE, Arnesjø B, Kambestad B. Carcinoma of the pancreas and papilla of Vater: presenting symptoms, signs, and diagnosis related to stage and tumour site. A prospective multicentre trial in 472 patients. Norwegian Pancreatic Cancer Trial. Scand J Gastroenterol. 1992;27(4):317–325.
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7(5):189–197.
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850.
- Sharma M, Holmes HM, Mehta HB, et al. The concomitant use of tyrosine kinase inhibitors and proton pump inhibitors: prevalence, predictors, and impact on survival and discontinuation of therapy in older adults with cancer. Cancer. 2019;125(7):1155–1162.
- Ben QW, Liu J, Sun YW, et al. Cigarette smoking and mortality in patients with pancreatic cancer: a systematic review and meta-analysis. Pancreas. 2019;48(8):985–995.
- Shi YQ, Yang J, Du P, et al. Effect of body mass index on overall survival of pancreatic cancer: a meta-analysis. Medicine. 2016;95(14):e3305.
- Li G, Taljaard M, Van den Heuvel ER, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46(2):746–755.
