Abstract
Background
For patients undergoing allogeneic hematopoietic stem cell transplant (HSCT) for acute myeloid leukemia (AML), disease relapse remains the most common reason for transplant failure and patient death. Recent randomized controlled trials (RCTs) have aimed to reduce the risk of relapse by means of post-transplant maintenance therapy.
Methods
We performed a systematic review and meta-analysis of RCTs comparing the efficacy and safety of maintenance with observation or placebo in patients with AML after allogeneic HSCT. We searched Cochrane Library, PubMed and conference proceedings up to Febuary 2021.
Results
Our search yielded five trials including 736 patients. Maintenance therapy consisted of tyrosine kinase inhibitors (TKIs) in 3 studies (sorafenib 2 studies; midostaurin 1 study) and hypomethylating agents (HMAs) in 2 studies (decitabine and azacytidine 1 study each). Maintenance therapy was associated with an improved overall survival (OS), HR = 0.61 (95% CI 0.47–0.80). Subgroup analysis revealed advantage in OS with either TKI or HMA maintenance. Relapse free survival (RFS) was also improved in the maintenance arm compared with the control arm HR = 0.51(95% CI 0.40 − 0.66). There was no difference between the two arms in overall grade 3/4 adverse events or overall infections, in grade 3/4 infections, or in acute and chronic graft versus host disease.
Conclusions
Our meta-analysis shows that post-transplant maintenance therapy in AML patients is effective in improving RFS and OS, with a satisfactory safety profile.
Background
Allogeneic hematopoietic stem cell transplant (HSCT) is standard frontline therapy for patients with acute myeloid leukemia (AML) when the risk for relapse overweighs projected transplant related morbidity and mortality [Citation1]. Taking into consideration excess morbidity and mortality (non - relapse mortality, NRM) inferred by the transplant process, this treatment modality is reserved for patients with sufficiently high relapse risk.
Disease relapse remains the most common reason for transplant failure and patient death, and treatment of leukemia relapse remains extremely challenging [Citation2]. Different strategies have been explored, aiming to reduce the risk of post-transplant relapse without inflicting excess toxicity. Pre-transplant conditioning intensity, as well as drug choice and duration of immunosuppression for graft versus host (GVHD) prophylaxis have been shown to modulate graft versus leukemia (GVL) effect and influence relapse rates [Citation3]. Post-transplant therapeutic strategies have also been examined to this end, including prophylactic donor lymphocyte infusion (DLI), and pharmacological maintenance treatment, mainly with hypomethylating agents (HMAs) and tyrosine kinase inhibitors (TKIs) [Citation3,Citation4]. Post-transplant maintenance could potentially slow down leukemia progression sufficiently until the desired immunological GVL effect of the transplant has begun, as well as augment this protective anti-leukemic effect [Citation5].
Recently, several prospective RCTs evaluating the role of maintenance treatment for patients with AML after allogeneic HSCT have been published. The aim of the present study was to evaluate the efficacy and safety of maintenance therapy after allogeneic HSCT in this setting.
Materials and methods
We searched PubMed until February 2021, The Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library, until Febuary 2021, and the following conference proceedings: Annual Meeting of the American Society of Hematology (till December 2020), Annual Meeting of the American Society of Clinical Oncology Annual Meeting (till May 2020), Annual Meeting of the European Hematology Association (till June 2020), Annual Meeting of the European Society of Blood and Marrow Transplantation (till August 2020) and Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR (Till February 2020). We cross‐searched the terms ‘acute myeloid leukemia’ and similar terms, ‘allogeneic transplant’ and ‘maintenance’ and similar terms. For PubMed, we added the Cochrane highly sensitive search term for identification of clinical trials. In addition, we scanned references of all included trials and reviews identified for additional studies.
Study selection
We included all randomized controlled trials (RCTs) that compared maintenance therapy with observation or placebo in patients with AML after allogeneic HSCT.
Data extraction and quality assessment
Two reviewers (O.P., R.G.) independently extracted data regarding case definitions, characteristics of patients, and outcomes from included trials. In the event of disagreement between the 2 reviewers regarding any of the above, a third reviewer (A.G.) extracted the data. Data extraction was discussed, and decisions were documented.
Two reviewers independently assessed the trials for the following domains: allocation concealment, generation of the allocation sequence, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data reporting, and selective outcome reporting. We made critical assessment separately for each domain and graded it as low, unclear, or high risk for bias according to the criteria specified in the Cochrane Handbook version 5.1.0.7 [Citation6].
Outcome measures
Primary outcome was overall survival (OS). Secondary outcomes included relapse free survival (RFS), relapse rate and safety (including adverse events and GVHD).
Relapse free survival was defined as time from transplant [Citation7,Citation8] or from randomization [Citation9] to either AML relapse or death from any cause, whatever occurred first
Data synthesis and statistical analysis
Hazard ratios (HRs) and variances for time‐to‐event outcomes were estimated and pooled in Review Manager (version 5.3 for Windows; The Cochrane Collaboration, Oxford, UK). An OR less than 1.0 was in favor of maintenance therapy.
Relative risks (RRs) and 95% confidence intervals (CIs) for dichotomous data were estimated using the Mantel‐Haenszel method.
We assessed heterogeneity of trial results by the chi test of heterogeneity, and the I2 statistic of inconsistency [Citation10]. Statistically significant heterogeneity was defined as p less than 0.1 or an I2 statistic greater than 50%. We conducted the meta‐analysis using a fixed‐effect model (FEM), and in case of high heterogeneity, we used random‐effects model (REM).
We planned to perform some subgroup analyses, according to type of maintenance therapy (HMA and TKI post-transplant maintenance) and according to MRD status.
Results
Description of trials
The literature search yielded 284 trials, of which 22 were considered as potentially relevant. Seventeen were excluded for various reasons (). Five trials fulfilled the inclusion criteria, all published in peer-reviewed journals. The trials were conducted between the years 2009 and 2018. Trial characteristics are presented in . Post-transplant maintenance consisted of TKIs in three trials: sorafenib – two studies [Citation9,Citation11]; midostaurin – one study [Citation7], and hypomethylating agents (HMAs) in two studies: decitabine and azacytidine – one study each [Citation8,Citation12]. In the trial by Gao et al. patients in the treatment arm received decitabine with GCSF as part of the protocol.
Figure 1. Flow diagram of publications identified for study and exclusions. RCT: randomized control trial.
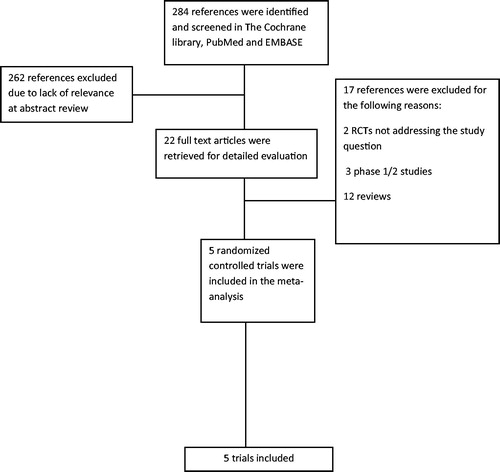
Table 1. Characteristics of included trials.
Patient characteristics
Seven-hundred and thirty six patients were included in five trials. Three trials included patients with FLT3-ITD mutated AML (the trials with TKI maintenance), and two trials included patients with high risk AML, defined as AML with poor genetic abnormalities, primary refractory AML, relapsed AML, or secondary AML in the trial by Gao et al. [Citation12]. In the trial by Oran et al. high risk features included induction failure, relapsed disease, second remission or beyond at HSCT, or first remission with either chromosome 5 or 7 abnormalities, complex karyotype or FLT3 mutations [Citation8]. The latter trial also included patents with high risk MDS. Median age of patients ranged between two and 76 years old.
Transplant procedure
Most patients underwent allogeneic HSCT at complete remission (N = 120, 84%). In the two trials with MRD information [Citation9,Citation12], 61% (N = 157) of patients were MRD negative at transplantation. Myeloablative conditioning was used for the majority of patients included in the trials (89%, N = 644). Data regarding transplant procedure is presented in .
Table 2. Transplant characteristics of included trials.
Risk of bias of included trials
Three trials were judged at low risk of selection bias [Citation9,Citation11,Citation12]. In the other two trials [Citation7,Citation8], methods of allocation concealment and generation were not reported. Blinding of patients and personnel was done in one trial [Citation9]. All five trials were judged at low risk of attrition bias, and at low risk of reporting bias as clinically important outcomes including overall survival were well addressed.
Primary outcome
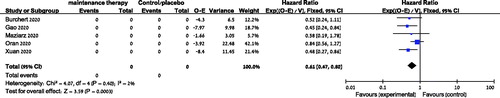
Data from five trials were available for analysis of OS [Citation7–9,Citation11,Citation12]. Maintenance therapy after allogeneic HSCT was associated with an improved OS, OR = 0.61 (95% CI 0.47–0.80, I2=2%, 547 patients, ). Subgroup analyses by type of maintenance therapy also showed advantage in OS with either TKI or HMA maintenance [HR = 0.50 (95% CI 0.33–0.77, 3 trials, 345 patients, Figure S1) and HR = 0.69 (95% CI 0.49–0.98, 2 trials, 391 patients, Figure S2), respectively]. Survival advantage was observed both in trials in which maintenance was initiated before day +60 post transplant [Citation7,Citation11], as well as trials in which maintenance was initiated after day +60 [Citation9,Citation12] [(HR = 0.50 (95% CI 0.30–0.84, 2 trials, 262 patients) and (HR = 0.47 (95% CI 0.29–0.77, 2 trials, 287 patients), respectively].Regarding subgroup analysis by MRD, there was insufficient data to conduct this analysis. The two trials which reported MRD-dependent outcomes did not report MRD-dependent survival [Citation9,Citation12].
Secondary outcomes
Data from four trials were available for analysis of all-cause mortality (ACM) [Citation7,Citation9,Citation11,Citation12]. Maintenance therapy after allogeneic HSCT was associated with reduced ACM, OR = 0.47 (95% CI 0.31–0.70, I2=0%, 547 patients).
Data from five trials was available for RFS analysis and showed improved RFS in the maintenance group compared with the control arm HR = 0.51 [95% CI 0.40−0.66], 736 patients (). Relapse rate was significantly decreased in the maintenance arm compared to the control arm, RR = 0.41 (95% CI 0.20–0.88, 4 trials, 668 patients).
Figure 3. Relapse free survival of patients treated with post-transplant maintenance compared to no maintenance. CI: confidence interval; O: observed; E: expected.
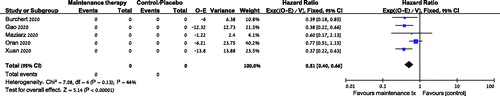
Two trials reported RFS according to MRD status. In the SORMAIN trial, patients who were MRD negative prior to transplant benefited most from maintenance therapy in terms of RFS: none of the MRD negative patients in the maintenance arm relapsed or died during follow up, whereas 5 of 12 MRD negative patients in the placebo arm relapsed (p = 0.028) [Citation9]. Similarly, in the trial by Gao et al., patients with MRD negativity had the most benefit from maintenance therapy: there was a 2 year cumulative relapse rate of 5.9% in the MRD negative maintenance arm versus 31% in the MRD negative no-maintenance arm (HR 0.16, p < 0.01). The difference in relapse rate was less pronounced if the MRD was positive prior to transplant, with relapse rates in the maintenance and no-maintenance arms of 34.5 and 52.9%, respectively (HR 0.48, p = 0.05) [Citation12].
Safety
Three trials reported grade 3 or 4 adverse events. The risk of any grade 3 or 4 adverse events did not increase with the addition of maintenance after allogeneic HSCT, RR = 1.0 (95% CI 0.83–1.20, 464 patients).
Acute/chronic GVHD
No difference was noted between the two arms regarding grade 2–4 acute GVHD, mild-moderate chronic GVHD or severe chronic GVHD.
Infections
There was no difference between the maintenance and control arms in the rate of all infections or grade 3 or 4 infections [(RR = 0.98 (95% CI 0.83–1.16, I2=0%, three trials, 585 patients) and (RR = 0.96 (95% CI 0.68–1.36, three trials, 464 patients), respectively].
Hematological toxicity
There was no difference between the two arms in grade 3 or 4 thrombocytopenia or in grade 3 or 4 neutropenia.
Discussion
This systematic review and meta-analysis shows that post-transplant maintenance therapy in AML patients improves outcomes, including RFS and OS, without a signal of excess toxicity.
Maintenance therapy following an initial induction phase has been incorporated into management schemes of several hematological malignancies in recent years For example, in follicular lymphoma, rituximab maintenance is an acceptable option following initial chemoimmunotherapy at first line and at relapse [Citation13], based on improved PFS and OS which were demonstrated in a systematic review and meta-analysis by Vidal et al [Citation14]. In multiple myeloma, maintenance therapy with either immunomodulatory drugs or proteosome inhibitors is standard of care for patients after autologous HSCT, with drug selection according to risk stratification [Citation15]. A recent meta-analysis showed improved OS for patients with Philadelphia positive acute lymphoblastic leukemia who received TKI maintenance after allogeneic HSCT [Citation16]. MRD status prior to transplant was associated with improved outcomes for second generation TKIs.
In AML, maintenance therapy of histamine dihydrochloride and interleukin-2 improved leukemia-free survival after initial induction and consolidation therapy in a phase 3 RCT without improvement in OS [Citation17]. More recently, large RCTs of post-remission maintenance with the HMA azacitidine in both subcutaneous [Citation18] and oral [Citation19] forms, have shown improved RFS in older patients not planned to undergo allogeneic HSCT. In the study by Wei et al. an improved OS with maintenance azactidine was observed as well [Citation19].
A mechanistic rationale for potential benefit exists for post-transplant maintenance in AML, which could temporarily impede leukemia progression until the transplant-induced immunological GVL begins. Furthermore, maintenance therapies could also augment this effect [Citation5,Citation20]. This hypothesis has been strengthened by the finding that sorafenib can promote GVL through production of IL-15 in both mouse and human cells [Citation21]. The ideal post-transplant maintenance regimen would be one which provides maximal anti-leukemic effects, without impeding donor chimerism or GVL.
Recent advances in the understanding of AML molecular biology has brought the opportunity for novel therapeutics at different treatment stages. The TKI sorafenib has been added to intensive induction chemotherapy for newly diagnosed AML patients, with varying results. In a study of patients older than 60 years, the addition of sorafenib did not confer improved outcomes [Citation22]. Yet, a study in younger patients showed improved EFS and RFS with the addition of sorafenib to induction chemotherapy [Citation23]. In the RATIFY trial, midostaurin conferred both EFS and survival benefit when incorporated into the induction, consolidation and maintenance phases of treatment for FLT3 mutated AML [Citation24]. In the present study, three trials of TKI maintenance were incorporated into the analysis, two with sorafenib and one with midostaurin. Subgroup analysis revealed a highly significant improved OS with a HR of 0.5 (95% CI 0.33–0.77) with TKI maintenance.
HMAs have revolutionized first line treatment for AML in certain patient populations, including older patients previously deemed unfit for available curative chemotherapeutics [Citation25]. Two trials with HMA maintenance (one each with decitabine and azacytidine) were included in the present study, and subgroup analysis revealed a significantly improved OS with a HR of 0.69 with HMA maintenance.
In 2016, Rashidi et al. presented a thorough review of available data regarding RCTs of maintenance therapy in AML at the time [Citation20]. Due to the lack of prospective RCTs for maintenance therapy for AML after allogeneic HSCT, the authors recommendation was to encourage patients to participate in such clinical trials. More recently, Bewersdorf et al. presented in abstract form, a meta-analysis of both RCTs and retrospective studies of maintenance therapy following allogeneic HSCT for AML or MDS [Citation26]. Most studies included in this analysis were retrospective, and methodological diversity hampered firm conclusions. Three of the five trials included in the present meta-analysis were also included in the report by Bewersdorf et al. in abstract form. In our analysis we had the complete data available from these three trials, which have since been published in peer-review journals. Furthermore, in our meta-analysis we included only prospective RCTs, enhancing the level of evidence of our findings.
Two trials included in our study reported data regarding MRD status prior to transplant, one using flow cytometry [Citation12], and the other molecular markers for NPM1 or FLT3 [Citation9] for MRD testing. In both studies, pre-transplant MRD negativity was associated with increased benefit of post-transplant maintenance. In the SORMAIN study, MRD positivity post-transplant was also associated with improved benefit from sorafenib [Citation9]. These subgroup analyses were conducted on a relatively small number of patients. Furthermore, the effect of MRD status on RFS was reported in the SORMAIN, whereas relapse rate was the MRD-dependent outcome in the study by Gao et al., making subgroup meta-analysis according to MRD status unfeasible. Given the possible role of MRD-driven preemptive therapies after allogeneic HSCT in patients with AML [Citation3], and the preliminary data from the aforementioned two trials included in our meta-analysis, it is reasonable to assume that similar MRD-driven therapeutic decisions could be applied to post-transplant maintenance.
The most important limitation of our study is the variability between the different trials, mainly the different type of drug used as maintenance therapy. Furthermore, also trials conducted with the same drug had methodological difference, including both drug administration regimen, as well as patient and transplant characteristics. Nonetheless, we were able to demonstrate a significant improvement in efficacy outcomes without signals for added toxicity across the various included trials. Our meta-analysis provides a proof of concept that biological agents have a role in maintenance for AML
In conclusion,our meta-analysis shows that post-transplant maintenance therapy in AML patients is effective in decreasing relapse rate and improving RFS and OS, with a satisfactory safety profile. Future prospective trials could aim to incorporate a broader spectrum of AML patients who will benefit from various post-transplant maintenance regimens.
Supplemental Material
Download MS Word (50.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Rashidi A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol. 2018;181(1):27–37.
- Zeiser R, Beelen DW, Bethge W, et al. Biology-driven approaches to prevent and treat relapse of myeloid neoplasia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(4):e128–e140.
- Antar AI, Otrock ZK, Abou Dalle I, et al. Pharmacologic therapies to prevent relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Front Oncol. 2020;10:596134.
- Sterling C, Webster J. Harnessing the immune system after allogeneic stem cell transplant in acute myeloid leukemia. Am J Hematol. 2020;95(5):529–547.
- Higgins JPT, Thomas J, Chandler J, editors. Cochrane handbook for systematic reviews of interventions. Version 6.0 (updated July 2019). Hoboken (NJ): Cochrane; 2019.
- Maziarz RT, Levis M, Patnaik MM, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021;56(5):1180–1189.
- Oran B, de Lima M, Garcia-Manero G, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020;4(21):5580–5588.
- Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–1212.
- Gao L, Zhang Y, Wang S, et al. Effect of rhG-CSF combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: an open-label, multicenter, randomized controlled trial. J Clin Oncol. 2020;38(36):4249–4259.
- Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–327.
- Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance improves overall survival of patients with follicular lymphoma-Individual patient data meta-analysis. Eur J Cancer. 2017;76:216–225.
- Kumar SK, Callander NS, Hillengass J, et al. NCCN guidelines insights: multiple myeloma, version 1.2020. J Natl Compr Canc Netw. 2019;17(10):1154–1165.
- Warraich Z, Tenneti P, Thai T, et al. Relapse prevention with tyrosine kinase inhibitors after allogeneic transplantation for philadelphia chromosome-positive acute lymphoblast leukemia: a systematic review. Biol Blood Marrow Transplant. 2020;26(3):e55–e64.
- Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88–96.
- Huls G, Chitu DA, Havelange V, Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON), et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457–1464.
- Wei AH, Dohner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526–2537.
- Rashidi A, Walter RB, Tallman MS, et al. Maintenance therapy in acute myeloid leukemia: an evidence-based review of randomized trials. Blood. 2016;128(6):763–773.
- Mathew NR, Baumgartner F, Braun L, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24(3):282–291.
- Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–3118.
- Rollig C, Serve H, Huttmann A, Study Alliance Leukaemia, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464.
- Gardin C, Dombret H. Hypomethylating agents as a therapy for AML. Curr Hematol Malig Rep. 2017;12(1):1–10.
- Bewersdorf JP, Tallman MS, Cho C, et al. Safety and efficacy of maintenance treatment following allogeneic hematopoietic cell transplant in acute myeloid leukemia and myelodysplastic syndrome – a systematic review and meta-analysis. Blood. 2020;136(Supplement 1):34–35.