Abstract
Background
The European Society for Therapeutic Radiology and Oncology Advisory Committee in Radiation Oncology Practice (ESTRO-ACROP) recently released new contouring guidelines for postmastectomy radiation therapy (PMRT) after implant-based reconstruction (IBR). As compared to prior ESTRO guidelines, the new guidelines primarily redefined the chest wall (CW) target to exclude the breast prosthesis. In this study, we assessed the impact of these changes on treatment planning and dosimetric outcomes using volumetric-modulated arc therapy (VMAT) and proton pencil-beam scanning (PBS) therapy.
Methods
We performed a treatment planning study of 10 women with left-sided breast cancer who underwent PMRT after IBR. All target structures were delineated first using standard (ESTRO) breast contouring guidelines and then separately using the new (ESTRO-ACROP) guidelines. Standard organs-at-risk (OARs) and cardiac substructures were contoured. Four sets of plans were generated: (1) VMAT using standard ESTRO contours, (2) VMAT using new ESTRO-ACROP contours, (3) PBS using standard contours, and (4) PBS using new contours.
Results
VMAT plans using the new ESTRO-ACROP guidelines resulted in modest sparing of the left anterior descending coronary artery (LAD) (mean dose: 6.99 Gy standard ESTRO vs. 6.08 Gy new ESTRO-ACROP, p = 0.010) and ipsilateral lung (V20: 21.66% vs 19.45%, p = 0.017), but similar exposure to the heart (mean dose: 4.6 Gy vs. 4.3 Gy, p = 0.513), with a trend toward higher contralateral lung (V5: 31.0% vs 35.3%, p = 0.331) and CW doses (V5: 31.9% vs 35.4%, p = 0.599). PBS plans using the new guidelines resulted in further sparing of the heart (mean dose: 1.05 Gy(RBE) vs. 0.54 Gy(RBE), p < 0.001), nearly all cardiac substructures (LAD mean dose: 2.01 Gy(RBE) vs. 0.66 Gy(RBE), p < 0.001), and ipsilateral lung (V20: 16.22% vs 6.02%, p < 0.001).
Conclusions
PMRT after IBR using the new ESTRO-ACROP contouring guidelines with both VMAT and PBS therapy is associated with significant changes in exposure to several cardiopulmonary structures.
Introduction
The rates of PMRT following breast reconstruction have been rising over time [Citation1]. However, these cases remain complicated to plan. Radiation oncologists have historically used field-based planning techniques for PMRT. In the setting of immediate IBR, there are concerns about the ability to adequately dose the chest wall and internal mammary nodes (IMNs) without causing undue exposure to adjacent anatomic structures, including the heart, lungs, and contralateral breast/chest wall [Citation2]. Thus, there has been a shift toward more conformal treatment modalities in this setting, including VMAT and proton therapy [Citation3,Citation4].
In 2019, ESTRO-ACROP published new consensus guidelines for PMRT target volume delineation after IBR [Citation5]. Seeking to take advantage of modern volume-based planning techniques, these international guidelines utilized location-specific recurrence data from the Danish Breast Cancer Cooperative Group cohort as well as lymphatic drainage pathways to delineate high-risk anatomical regions after mastectomy [Citation6,Citation7]. Whereas prior field-based techniques using historical contouring guidelines generally included the implant and chest wall musculature [Citation8–10], the new ESTRO-ACROP guidelines removed these structures from the clinical target volume (CTV) [Citation5,Citation11]. Though they did not publish the dosimetric outcomes with their updated guidelines, a planning exercise conducted at several European centers suggested that these more limited target volumes could lead to changes in the radiation dose distribution, potentially improving treatment toxicity [Citation12].
In this context, the main objectives of this study were two-fold. First, we assessed the feasibility of generating volume-based treatment plans using the new ESTRO-ACROP consensus contouring guidelines for women undergoing PMRT after IBR using both VMAT and proton PBS treatment modalities. Second, we compared the dosimetric outcomes of these plans to those generated from standard ESTRO contouring guidelines, assessing differences in target coverage and normal tissue exposure.
Material and methods
In this study, 40 radiation plans were generated from planning CT scans of 10 women who underwent left-sided PMRT after immediate IBR. Initially, all target OARs were delineated using standard PMRT consensus contouring guidelines published by ESTRO [Citation10] (henceforth referred to as the ‘standard’ guidelines). Then, the second set of contours were separately created using the new ESTRO-ACROP guidelines [Citation5] (referred to as the ‘new’ guidelines). Next, 4 sets of plans were generated for each patient: (1) a VMAT plan using standard contours, (2) a VMAT plan using new contours, (3) a PBS plan using standard contours, and (4) a PBS plan using new contours. Finally, after optimization, doses to target structures and OARs were compared across all plans. This project was considered exempt from IRB approval at our institution.
Patient information
All 10 women included in this study were diagnosed with cT1-T3N + left-sided breast cancer and were treated with mastectomy and immediate IBR. Five women had pre-pectoral implant reconstructions, while 5 had subpectoral implant reconstructions. Patients were immobilized supine on a breast board with their arms abducted and externally rotated over their heads. Non-contrast computed tomography (CT) scans were performed in this position, imaging each patient from their cricoid cartilage to their mid-abdomen in 2.5 mm slices. CT scans were obtained during normal respiratory movement and deep inspiratory breath-hold was not employed per our institutional standard for patients being treated with PBS [Citation13].
Contouring
Two radiation oncologists delineated all targets and OARs for the 10 patients included in this study using both the standard and new guidelines. Target structures included the CW, IMNs, three levels of axillary lymph nodes including Rotter’s nodes, and the supraclavicular fossa. OARs included the contralateral breast, thyroid gland, esophagus, lungs, and heart. For this study, we additionally utilized a cardiac contouring atlas to delineate the left ventricle (LV), right ventricle (RV), left atrium (LA), right atrium (RA), right coronary artery (RCA), and LAD [Citation14]. The breast implant was contoured for all patients but was not considered a target or an OAR.
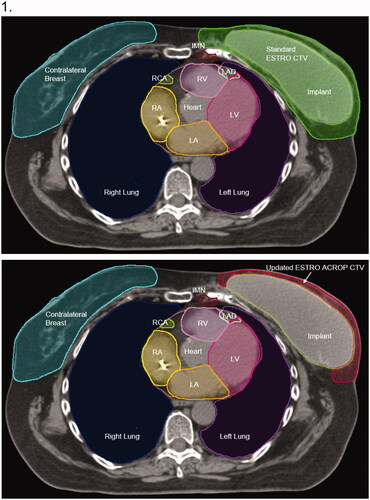
The major difference between the standard and new guidelines is in the definition of the CW CTV. In both guidelines, the CW begins caudally as high as the inferior aspect of the sternoclavicular joint and extends inferiorly to the level of the prior breast tissue. Medially, the CW begins just lateral to the perforating mammary vessels and extends laterally to the mid-axillary line, anterior to the lateral thoracic artery. Per the standard guidelines, within these boundaries, the CW encompasses all of the soft tissue anterior to the pectoralis, costal, and intercostal muscles, stopping 5 mm under the surface of the skin [Citation10]. Alternatively, citing that in the absence of high-risk features the majority of local recurrences after mastectomy occur in the residual subcutaneous tissues, the new guidelines recommend a more limited CW volume [Citation5]. For both sub-pectoral and pre-pectoral implants, the CW includes the soft tissue anterior to the pectoral muscles, chest wall musculature, or implant, ventrally up to 3–5 mm under the surface of the skin. For patients status post subpectoral implant with high-risk factors or tumors adjacent to the dorsal fascia where it is not covered by the pectoralis major muscle, the region between the chest wall and the implant superior to the pectoralis major should also be included in the CTV [Citation5]. Likewise, for patients with pre-pectoral implants and high-risk factors, the region between the dorsal aspect of the implant and the underlying pectoral muscles/chest wall may also be included [Citation5]. Notably, none of the patients in this study had high-risk factors or tumors adjacent to the chest wall, and these dorsal regions of the CW CTV were not included. Supplemental Table 1 presents the boundaries of the CW structures utilized in this study. Notably, the axillary, IMN, and SCV nodal targets were contoured identically across guidelines.
presents representative axial slices of each contouring guideline for a single patient. Differences between the CW structures generated from each guideline were assessed by comparing volumes and the degree of overlap. Per institutional practice, a PTV expansion of 3 mm was used for VMAT plans; PTV expansions were not utilized for PBS plans.
Figure 1. Representative CW contours using standard ESTRO consensus guidelines (green) and updated ACROP consensus guidelines (red). Images are from the same axial slice of the planning CT scan for a single patient with a pre-pectoral implant at the T8 level (Panel 1) and carina (Panel 2).
Radiation planning
Treatment plans were designed to simultaneously achieve adequate coverage of all targets while maximally sparing dose to all OARs. Prescription doses for all targets were 45.0–50.4 Gy or Gy(RBE) in daily 1.8 Gy or Gy(RBE) fractions for all plans. Plans were rendered to achieve a mean dose to each target of the prescription dose, or until maximum doses were achieved. In cases where normal tissue dose constraints or plan homogeneity made target coverage difficult, target goals were prioritized.
To ensure a consistent comparison, a standard institutional dosimetric algorithm was used for all plans [Citation15]. For proton plans, initial target goals were defined as follows: 45 Gy(RBE) mean dose to the CW, IMNs, supraclavicular nodes, and axillary levels 2 and 3, followed by a 5.4 Gy(RBE) mean boost to the CW and IMNs. Then the minimum dose to the level 1 axillary nodes was set at 40 Gy(RBE) and the maximum dose to the CW skin (3 mm thickness) was set at 49 Gy(RBE). Next, OAR constraints were locked at the following levels: 1 Gy(RBE) mean heart dose (MHD), 1.5 Gy(RBE) mean LAD dose, 7 Gy(RBE) mean ipsilateral lung dose, 40 Gy(RBE) maximum dose to the esophagus, and 45 Gy(RBE) maximum dose to the thyroid. After the above planning constraints were set, the following planning objectives were weighed: (1) minimize maximum dose to the patient (notably, hot spots were limited to less than 103.2% of prescription for PBS plans), (2) minimize underdosing of the individual targets at their respective prescription doses, and (3) minimize dose above 45 Gy(RBE) to the supraclavicular region and axillary level 2 and 3 nodes. Proton plans were generated with the Astroid planning system (.decimal, LLC), using a multicriteria optimization (MCO) Pareto-surface navigation to arrive at the most clinically suitable solution. The proton treatment machine considered for this work used energies from 70 MeV to 250 MeV, spot sizes between 3 and 7 mm, and a 45 mm range shifter to ensure potential delivery to the patient’s surface. A single en-face beam targeting the CW and nodal structures was used. Further details on the PBS treatments have been further described in a previous publication [Citation15].
For VMAT plans, every case shared the same initial MCO Pareto-surface generation as described above (Supplemental Table 2 lists all of the optimization criteria for both VMAT and PBS plans). Per recommendations, VMAT plans did not utilize synthetic bolus. Hot spots were limited to less than 110% of the prescription dose. Once an optimal solution was achieved through user-specified navigation, plans were further optimized with additional customized constraints using a standard optimization environment. Such optimization ensured high plan quality while accounting for unique anatomical differences and treatment planning goals. VMAT plans were generated using the Raystation planning system version 8 A (RaySearch Laboratories, Stockholm, Sweden) with 6 MV beam energies via a Varian TrueBeam linear accelerator. Though the VMAT plans were not actually used to treat any patients in this study, they appeared readily deliverable with mean monitor unit counts of 428.5 per fraction for standard ESTRO plans and 441.8 per fraction for updated ESTRO-ACROP plans.
Statistical analysis
We compared dosimetric goals between the standard and new guidelines within each treatment modality using several a priori determined criteria. The main target metrics included the dose received by 95% (D95) of the volume of the CW and regional nodal structures. OAR criteria included the following: (1) the mean dose received by the heart and cardiac substructures (2) the dose received by 1 cc of the volume (D1cc) of the heart and cardiac substructures, (3) the volume of the heart and cardiac substructures receiving at least 5 Gy(RBE) (V5), and (4) the volume of the ipsilateral lung, contralateral lung, and contralateral CW receiving either 5 (V5), 10 (V10), or 20 Gy(RBE) (V20). Dose-volume histograms were created for all plans and Wilcoxon tests were applied to compare the individual metrics across all 10 patients.
Results
Target delineation
A total of 20 left-sided CW targets were generated among 10 patients using first the standard (ESTRO) and then the new (ESTRO-ACROP) guidelines. As the new guidelines recommend excluding the implant from contoured volumes, the CW target structures largely consisted of a thin margin of subcutaneous tissue anterior to the implant. Consequently, the mean CW volume was significantly smaller when using the new guidelines (mean volume 999.2 mL with standard guidelines vs. 317.1 mL with new guidelines, p < 0.001). Likewise, the mean anterior-posterior thickness of the tissue included in the CW at the level of the nipple-areolar complex was smaller with the new guidelines (5.3 cm vs. 0.3 cm, p < 0.001).
Target coverage
As shown in , target coverage was equivalent for all CW and nodal PTVs for all patients, irrespective of the contouring guideline. For VMAT plans, the mean CW D95 was 49.2 Gy with standard guidelines and 50.1 Gy with the new guidelines. For PBS plans, mean CW D95 values were identical across guidelines at 47.8 Gy(RBE). In general, PBS plans were able to achieve lower maximum percent doses and provided a more homogeneous dose across CWs. Using the updated guidelines, there were no differences in mean CW D95 between pre- and sub-pectoral implants (p = 0.873). Likewise, there were no differences in coverage for the nodal targets when using standard or new contouring guidelines.
Table 1. Mean chest wall and nodal target coverage dose metrics.
Organs at risk
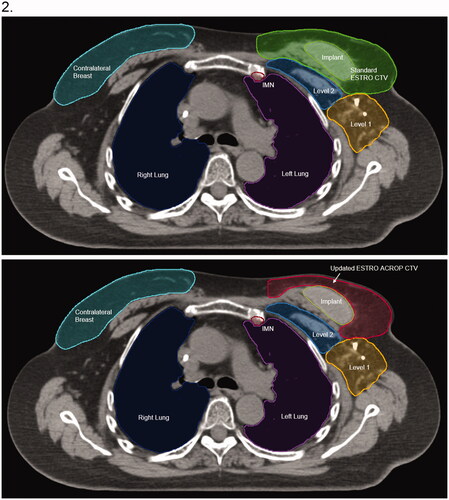
Defining the CW according to the new guidelines was associated with similar to improved doses to multiple critical avoidance structures. displays representative isodose distributions using each contouring guideline and treatment modality, and presents the dosimetric outcomes for the regional OARs.
Figure 2. Representative isodose distribution from treatment plans using (A) VMAT with standard ESTRO guidelines, (B) VMAT with updated ACROP guidelines, (C) Proton PBS with standard ESTRO guidelines, and (D) Proton PBS updated ACROP guidelines. The 100% relative dose corresponds to an absolute dose of 50.4 Gy (RBE). All plans are for a single patient with pre-pectoral implant at the T8 level (Panel 1) and carina (Panel 2).
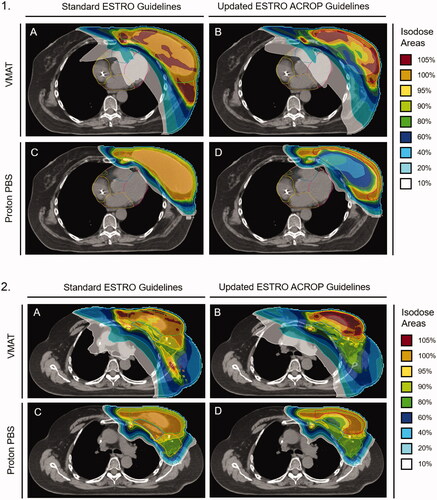
Table 2. Organ-at-risk dosimetrics for VMAT and PBS plans using standard ESTRO and updated ACROP contouring guidelines.
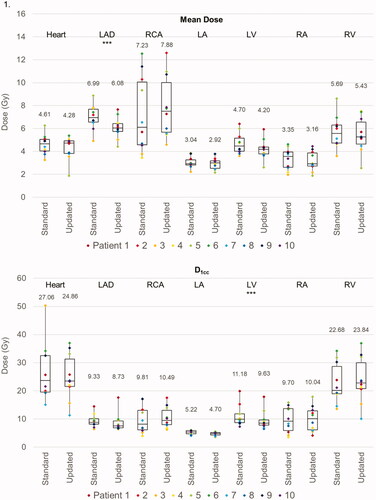
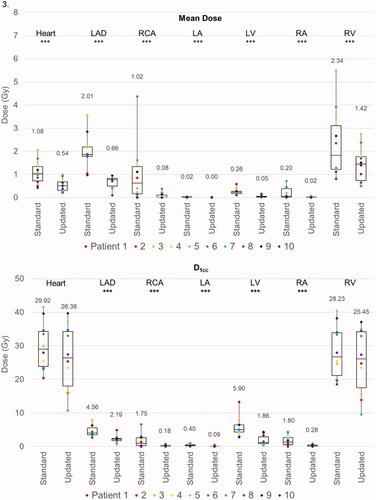
For VMAT, the average MHD was 4.6 Gy with the standard guidelines and 4.3 Gy with the new guidelines (p = 0.513). However, the mean LAD dose was significantly lower with the new guidelines (6.99 Gy with standard guidelines vs. 6.08 Gy with new guidelines, p = 0.010) as was the V5 metric (82.68% vs. 78.55%, p = 0.018). There were small but statistically significant differences in the LV D1cc (11.18 Gy vs. 9.63 Gy, p = 0.044) and V5 (35.4% vs. 29.0%, p = 0.048) between guidelines. There were no other statistically significant differences in dose metrics to other cardiac substructures, except for a small reduction in the LA V5 with the new guidelines (3.24% vs. 1.39%, p = 0.033).
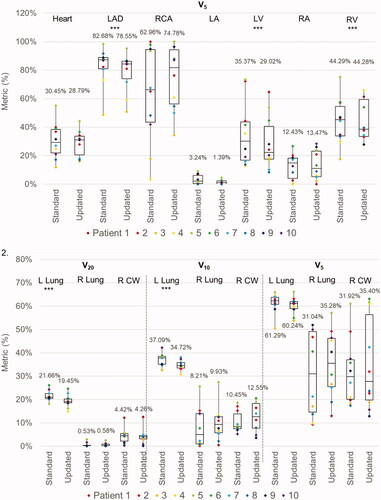
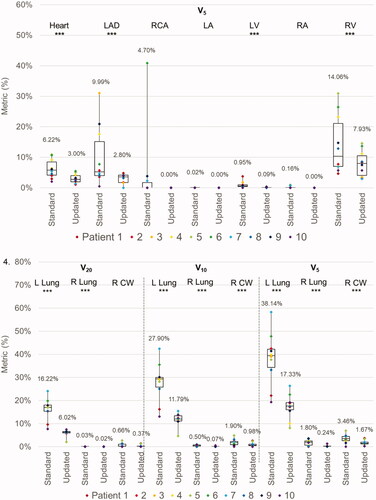
The new guidelines resulted in a small reduction in high doses to the ipsilateral lung (left lung V20: 21.66% with standard guidelines vs. 19.45% with new guidelines, p = 0.017). However, there was a trend toward a greater extent of low dose radiation to the contralateral lung (right lung V5: 31.0% vs. 35.3%, p = 0.331) and CW (right CW V5: 31.9% vs. 35.4%, p = 0.599). displays the difference in average OAR metrics between standard and new VMAT plans.
Figure 3. Box and whisker plots of the dose metrics for plans utilizing ‘standard’ ESTRO and ‘updated’ ESTRO-ACROP contouring guidelines. Panel 1: cardiac metrics (mean dose, D1cc, and V5) with VMAT plans. Panel 2: pulmonary and chest wall metrics (V20, V10, V5) with VMAT plans. Panel 3: cardiac metrics with PBS plans. Panel 4: pulmonary and chest wall metrics with PBS plans. The mean value for each dose metric is presented in the data label adjacent to each box and whisker plot. Metrics that were statistically significantly different between ‘standard’ and ‘updated’ plans are delineated with three asterisks (***) immediately below the metric. Individual metrics for each patient are presented as a color-coded marker overlying the appropriate box and whisker plot.
For PBS plans, the average MHD was 1.08 Gy(RBE) when using standard guidelines and 0.54 Gy(RBE) with new guidelines (p < 0.001). Similarly, the mean dose to all cardiac substructures was reduced with the new guidelines. Furthermore, the new guidelines were associated with significant reductions in the D1cc and V5 for several cardiac substructures, most notably the LAD (D1cc: 4.56 Gy(RBE) vs. 2.19 Gy(RBE), p = 0.003; V5: 9.99% vs. 2.80%, p = 0.048) and LV (D1cc: 5.90 Gy(RBE) vs. 1.86 Gy(RBE), p < 0.001; V5: 0.95% vs. 0.09%, p = 0.028). The new guidelines were associated with improved sparing of the ipsilateral lung (left lung V20: 16.22% vs. 6.02%, p < 0.001; V5: 38.14% vs. 17.33%, p < 0.001). However, as opposed to the VMAT plans, there was also a small but statistically significant reduction in low doses to the contralateral lung and CW (right lung V5: 1.80% vs. 0.24%, p = 0.004; right CW V5: 3.46% vs. 1.67%, p = 0.002). displays the difference in average OAR metrics between standard and new PBS plans. Of note, no differences in OAR metrics were observed when comparing pre- and sub-pectoral implants.
Discussion
ESTRO-ACROP recently published new contouring guidelines for PMRT after IBR, recommending that the implant be removed from the CTV [Citation5]. In light of this significant change, our study is, to our knowledge, the first to assess the impact of the new guidelines on the planning and dosimetric outcomes for patients undergoing PMRT with VMAT or PBS.
We found that the new guidelines were straightforward to implement. They did not require any changes to our institutional standard simulation process and only necessitated minimal updates for treatment planning. It is important to note, however, that the updated guidelines require an in-depth understanding of the surgical procedures performed and close attention to detail to ensure all areas at risk for recurrence are included in the CW CTV: differences in patient anatomy, type of reconstruction, and the presence or absence of high-risk factors may result in contours of various scope [Citation12,Citation16].
When using the new contouring guidelines, both VMAT and PBS plans yielded adequate target coverage metrics and acceptable dose heterogeneity. The new guidelines did result in a CW structure consisting of a thin strip of skin and subcutaneous tissue anterior to the implant, the thickness of which was highly dependent on the specifics of each patient’s reconstruction. In a few cases, when using the new guidelines, this tissue was so thin that trimming the recommended 3 to 5 mm below the surface of the skin was not feasible. Without utilizing synthetic bolus, the new guidelines risk undercovering a significant portion of the CW CTV. Indeed, we found that with a much smaller CW, the D95 of VMAT and PBS plans were highly sensitive to variability in treatment planning.
Dosimetrically, the primary difference between plans generated from the new (ESTRO-ACROP) contouring guidelines and those using the standard (ESTRO) guidelines was in the dose to regional OARs. Given that left-sided PMRT is associated with higher radiation doses to the heart and cardiac substructures [Citation17], and the more limited target volumes derived from the new guidelines could potentially have a greater relative effect on tissue sparing, we chose to limit our analysis to women undergoing left-sided PMRT.
Despite the smaller target volumes, we found that VMAT plans using the new guidelines resulted in similar mean radiation exposure to the heart and a non-significant reduction in the mean dose to the LV compared to standard guidelines. This may seem surprising considering the relative sparing of the CW abutting the heart with the new guidelines (). However, adequate IMN coverage required high doses of radiation along with the medial CW, increasing exposure to the underlying heart. Additionally, the greater conformality of VMAT plans hugging the narrow CW structure came at the expense of a larger low-dose radiation bath [Citation18], further limiting the benefit of the smaller target volume. Despite these challenges, VMAT plans utilizing the new guidelines were able to achieve significant reductions in the mean dose to the LAD.
Previous studies have shown that increasing radiation doses to the heart, left ventricle, and LAD are directly associated with long-term rates of high-grade coronary artery stenosis and acute coronary events [Citation19–22]. Darby et al. found that each 1 Gy increment in MHD was linked to a 7.4% increased risk of acute coronary events [Citation19], and Van den Bogaard et al. showed that the volume of the left ventricle exposed to 5 Gy after breast radiation was the strongest predictor of acute cardiac events [Citation22]. Similarly, Taylor et al. also showed that individual segments of the coronary arteries are sensitive to radiation in a dose-dependent manner [Citation21]. Given that VMAT plans using the new guidelines modestly spared the LAD but did not reduce the mean exposure to the heart or LV, they may not contribute to significant reductions in late cardiac toxicity. In contrast, PBS plans using the new guidelines showed significant further sparing of the heart and virtually all cardiac substructures. Indeed, given the close proximity of the IMNs to the underlying heart and considering the sharp dose fall-off with proton treatments, meticulous target definition in these cases is paramount in order to optimize target coverage and OAR sparing for each patient. Thus, using the new guidelines with PBS planning may represent an important step toward further increasing the long-term safety of PMRT.
The new guidelines resulted in VMAT and PBS plans with reduced exposure to the ipsilateral lung as compared to standard guidelines, which was most pronounced at higher dose levels (V20). Given the inherently large low dose bath, though, VMAT plans were unable to reduce low dose ipsilateral lung exposure. Further, the new guidelines tended to result in VMAT plans with increased doses to the contralateral lung and CW. This effect was primarily due to a relative increase in the weighting of lateral beam angles. Notably, this increased low-dose exposure may be associated with an increased risk of secondary malignancies and other complications [Citation23–25]. Alternatively, contralateral lung and CW exposure were further reduced when using PBS with the new guidelines. Differences, however, were small given the already negligible dosing achieved with standard guidelines.
A similar treatment planning exercise using updated ESTRO-ACROP contouring guidelines was performed at 13 centers in Europe on scans from 2 patients – one with right-sided and one with left-sided non-locally advanced breast cancer, both with sub-pectoral implants, simulated with DIBH [Citation12]. A single set of consensus contours were generated centrally for each patient, and a total of 35 plans were generated (18 right-sided and 17 left-sided plans). The left-sided plans included 9 field-in-field (FiF), 4 VMAT, 1 tomotherapy, 1 intensity-modulated radiation therapy (IMRT), and 2 hybrid plans. They reported an overall median left-sided CW CTV V95 of 91.4%, and a mean of 92.5% among the 4 VMAT plans; somewhat lower than the mean V95 of 99.0% for VMAT and 93.7% for PBS plans in our study. In their planning exercise, mean V90 values for the IMN CTV was 88.0% for FiF and 99.8% for VMAT plans – primarily reflecting differences in the ability to spare the underlying heart across treatment types – compared to 95.7% for VMAT and 94.0% for PBS plans in our study. Regarding OARs, their planning exercise reported a median MHD of 5.2 Gy for all left-sided plans, including a median of 5.2 Gy for FiF and 6.3 Gy for VMAT plans. Mean heart doses were lower in our study; 4.28 Gy for VMAT and 0.54 Gy for PBS plans. The authors of the planning exercise concluded that all evaluated planning techniques could be used with the updated contouring guidelines and that FiF specifically offered acceptable target coverage and OAR sparing. Our study further shows that VMAT and PBS plans provide acceptable target coverage while offering an opportunity to further reduce dosing to the heart and cardiac substructures. Clinicians should thus consider the potential advantages and disadvantages of each approach when optimizing treatments for their patients.
This study was strengthened by our utilization of multiple subjects with widely varying anatomy and both pre- and sub-pectoral implants. We believe that our results are likely generalizable to most women undergoing PMRT after IBR. However, there are a few limitations to consider. We did not evaluate the impact of altered dose fractionation on normal tissue exposure, although biologically equivalent doses are expected to be similar across different fractionation patterns. This study also did not evaluate the role of respiratory motion. All of the subjects included in this study were simulated without deep inspiration breath-hold (DIBH) reflecting standard practice at our institution for proton patients. We recognize that DIBH may reduce cardiopulmonary exposure [Citation26] and may alter the doses received by contralateral structures for VMAT. However, prior PBS studies have found no differences in cardiac exposure between breath-hold and non-breath hold techniques [Citation13]. Intra- and inter-fraction respiratory motion could also impact the dosimetric outcomes of each plan, but this effect is likely minor. Furthermore, we did not assess the impact of proton range uncertainty in our plans. However, when using typical treatment beam energies – which varied between 100 and 185 MeV in this study – this effect has been shown to be quite small [Citation15,Citation27].
Overall, there are important tradeoffs to consider when deciding which contouring guidelines to use for PMRT after IBR. The new ESTRO-ACROP guidelines result in VMAT plans that better spare the LAD and ipsilateral lung at the expense of increased low dose radiation to the contralateral lung and CW. In contrast, PBS plans resulted in further sparing of the heart, cardiac substructures, and ipsilateral lung without a concomitant increase in contralateral doses, potentially reducing the predicted risk for complications. However, the dosimetry of each plan varied significantly based on each patient’s anatomy. Thus, when optimizing treatment decisions for individual patients it may be worthwhile to create plans using both standard ESTRO and new ESTRO-ACROP guidelines and weighing each plan’s relative advantages and disadvantages.
Supplemental Material
Download MS Word (13.4 KB)Supplemental Material
Download MS Word (13.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Frasier LL, Holden S, Holden T, et al. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. 2016;2(1):95–101.
- Chen SA, Hiley C, Nickleach D, et al. Breast reconstruction and post-mastectomy radiation practice. Radiat Oncol. 2013;8(1):45–49.
- Kuo LC, Ballangrud ÅM, Ho AY, et al. A VMAT planning technique for locally advanced breast cancer patients with expander or implant reconstructions requiring comprehensive postmastectomy radiation therapy. Med Dosim. 2019;44(2):150–154.
- Jimenez RB, Hickey S, DePauw N, et al. Phase II study of proton beam radiation therapy for patients with breast cancer requiring regional nodal irradiation. J Clin Oncol. 2019;37(30):2778–2785.
- Kaidar-Person O, Vrou Offersen B, Hol S, et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol. 2019;137:159–166.
- Rudat V, Aziz Alaradi A, Mohamed A, et al. Tangential beam IMRT versus tangential beam 3D-CRT of the chest wall in postmastectomy breast cancer patients: a dosimetric comparison. Radiat Oncol. 2011;6(1):26.
- Jimenez RB, Goma C, Nyamwanda J, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol. 2013;107(2):213–217.
- White J, Tai A, Arthur D, et al. Breast cancer atlas for radiation therapy planning: consensus definitions 2 2 collaborators. NRG Oncology; 2020 [cited 2020 November 4]. https://www.nrgoncology.org/ciro-breast
- MacDonald S, Cahlon O. Breast Contouring RADCOMP Consortium v.3. NRG Oncology; 2016 [cited 2020 November 4]. https://www.nrgoncology.org/About-Us/Center-for-Innovation-in-Radiation-Oncology/Breast/RADCOMP-Breast-Atlas
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3–10.
- Leonardi MC, Spoto R, Miglietta E, et al. HALFMOON TomoTherapy (Helical ALtered Fractionation for iMplant partial OmissiON): implant-sparing post-mastectomy radiotherapy reshaping the clinical target volume in the reconstructed breast. J Cancer Res Clin Oncol. 2019;145(7):1887–1896.
- Kaidar-Person O, Nissen HD, Yates ES, et al. Postmastectomy radiation therapy planning after immediate implant-based reconstruction using the European Society for Radiotherapy and Oncology-Advisory Committee in Radiation Oncology Practice Consensus Guidelines for target volume delineation. Clin Oncol. 2021;33(1):20–29.
- Patel SA, Lu HM, Nyamwanda JA, et al. Postmastectomy radiation therapy technique and cardiopulmonary sparing: a dosimetric comparative analysis between photons and protons with free breathing versus deep inspiration breath hold. Pract Radiat Oncol. 2017;7(6):e377–e384.
- Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122(3):416–422.
- Depauw N, Batin E, Daartz J, et al. A novel approach to postmastectomy radiation therapy using scanned proton beams. Int J Radiat Oncol Biol Phys. 2015;91(2):427–434.
- Kaidar-Person O, Offersen BV, Boersma LJ, et al. A multidisciplinary view of mastectomy and breast reconstruction: understanding the challenges. Breast. 2021;56:42–52.
- Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72(2):501–507.
- Rastogi K, Sharma S, Gupta S, et al. Dosimetric comparison of IMRT versus 3DCRT for post-mastectomy chest wall irradiation. Radiat Oncol J. 2018;36(1):71–78.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998.
- Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30(4):380–386.
- Taylor C, McGale P, Brønnum D, et al. Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. J Clin Oncol. 2018;36(22):2288–2296.
- Van Den Bogaard VAB, Ta BDP, Van Der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35(11):1171–1178.
- Paganetti H, Depauw N, Johnson A, et al. The risk for developing a secondary cancer after breast radiation therapy: comparison of photon and proton techniques. Radiother Oncol. 2020;149:212–218.
- Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother Oncol. 2013;106(1):42–49.
- Hernandez MH, Zhang R, Sanders M, et al. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy. Jour Proton Ther. 2016;1(1):119.
- Bruzzaniti V, Abate A, Pinnarò P, et al. Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer. J Exp Clin Cancer Res. 2013;32(1):88.
- Ares C, Khan S, MacArtain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76(3):685–697.