Abstract
Purpose
We aimed to assess the incidence, clinical and biochemical course of immunotherapy-induced thyroiditis and its implication on patients’ survival, based on an extensive clinical experience from a tertiary cancer center.
Methods
Analyses were based on data from the electronic medical records of cancer patients treated with CPIs. Data included demographic characteristics, cancer type, Thyroid function tests (TFT), and survival.
Results
Thyroid function tests were available for 934 patients. After excluding patients with impaired baseline TFT or levothyroxine treatment, 754 euthyroid patients were included in the core analyses. Of those, 301 (39.9%) patients developed thyroid dysfunction (‘thyroiditis’). Thyroiditis was more prevalent in patients with renal cell carcinoma than other types of cancer. Survival rates were comparable in patients who developed thyroiditis and in those who did not. during the 5 years follow-up period, there was a non-significant trend toward improved survival in patients who developed TD in four predefined groups: melanoma, lung cancer, renal cell carcinoma, and transitional cell carcinoma. Nevertheless, we observed a highly significant survival benefit for patients with renal cell carcinoma who developed TD (HR = 0.19, 95% CI 0.06–0.60; p = 0.005).
Conclusions
Thyroiditis is common, often asymptomatic, and is more prevalent in patients treated with combinations of nivolumab and PD-L1 inhibitors, and in patients with renal cell carcinoma. Thyroiditis was associated with a trend for a survival benefit, particularly in patients with renal cell carcinoma.
Background
It is widely recognized that the extensive integration of immunotherapy has revolutionized cancer treatment. Immune checkpoint inhibitors (CPI) are synthetic, humanized antibodies directed against specific immune-inhibitory receptors: programmed cell death ligand 1 (PD-L1) located on tumor cells membranes, programmed cell death protein 1 (PD-1), and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) found on immune cells membranes [Citation1]. However, treatment with CPIs has introduced a new, heterogeneous class of immune-related adverse effects (irAEs). The skin, gastrointestinal tract, and endocrine glands are the most commonly affected organ systems [Citation2]. Among the endocrine irAEs, thyroid dysfunction (TD) is by far the most prevalent, occurring in 6–30% of CPI-treated patients in various studies [Citation3].
Commonly referred to as ‘CPI-induced thyroiditis’, until recently, the mechanism of CPI-induced TD was essentially implied from indirect evidence, including the hyperimmune setup, the clinical course, the decreased thyroid uptake, and increased uptake in 18fludeoxyglucose positron emission tomography-computed tomography (FDG-PET-CT) imaging. Direct histopathological correlates were broadly lacking [Citation4]. However, several recent observations provided solid evidence that CPI-induced TD involves infiltration of T-cell subpopulations and necrotic changes in the thyroid tissue [Citation5–7]. In addition, while the overall prevalence of anti-thyroid antibodies in patients with CPI-induced TD is low, it seems that CPI-induced TD occurs more frequently in patients with preexisting anti-thyroid peroxidase (TPO) antibodies [Citation8,Citation9].
Previous studies addressed the impact of TD following CPI and CPI-related AEs on disease course in patients with different malignancies, but the data is somewhat limited and inconsistent [Citation10–15]. Given the rapidly expanding use of CPIs, a broader perspective is desirable regarding factors that predict TD, its implications on the appropriate follow-up andmanagement of CPI-treated patients, and patients’ survival rates.
The present study, which is based on analyses of a large number of cancer patients treated with CPI in a single tertiary cancer center, was aimed to define further the impact of specific CPI agents and patients’ characteristics on the likelihood of developing TD and determine the impact of TD on survival in patients with various malignancies.
Patients and methods
Study population
This retrospective cohort study is based on the database of the Endo-Oncology Clinic in the Institute of Oncology at Sheba Medical Center, Israel, between January 2015 and October 2018. Patients were included if treated with at least one of the following immune checkpoint inhibitors (CPIs): the CTLA-4 inhibitor ipilimumab, the PD-1 inhibitors nivolumab, and pembrolizumab; the PD-L1 inhibitors atezolizumab, avelumab, and durvalumab, alone or in combination. In addition, patients treated as part of open-label clinical trials were included, whereas patients enrolled in placebo-controlled blinded trials were excluded.
Data were retrieved from the patients’ electronic records, including demographics, tumor type and site, treatment details and administration date, and previous thyroid hormone replacement therapy. Patients were included in the study only if normal baseline thyroid function tests (both fT4 and TSH) were recorded within 120 days before the first CPI administration and if not treated for thyroid dysfunction before initiation of a CPI treatment. Normal reference ranges for thyroid function tests were defined according to the reference ranges used at the Sheba Medical Center clinical endocrinology laboratory: serum TSH between 0.4 and 4.0 mIU/L, serum-free thyroxine (fT4) between 7 and 16 pmol/L, and serum-free triiodothyronine (fT3) between 3.3 and 7.2 pmol/L.
Subgroups’ definitions
CPI treatment regimens were categorized separately for each CPI drug and for the ipilimumab-nivolumab or any ipilimumab-PD-L1 inhibitor combinations. Administration of multiple CPI drugs within 30 days was considered concomitant [Citation16], whereas longer intervals were considered sequential.
Cancer types were grouped into the following sub-categories: lung cancer (n = 208), melanoma (n = 212), renal cell carcinoma (RCC; n = 27), transitional cell carcinoma (TCC; n = 52), and ‘other’ tumors that appeared in small numbers (n = 255; including adrenocortical carcinoma, basal cell carcinoma, breast carcinoma, cholangiocarcinoma, colorectal carcinoma, esophageal carcinoma, head and neck carcinoma, liver, meningioma, neuroendocrine tumors, pancreas, skin, small intestine, stomach, thymus, thyroid, and unknown primary).
Thyroid dysfunction (‘thyroiditis’) was defined as abnormal fT4 and/or elevated TSH, or the initiation of levothyroxine Rx replacement after CPI.
Statistical analysis
For continuous variables, results are presented as mean ± SD or median with inter-quartile range (IQR) and as percentages for categorical data. The student’s t-test was used for the comparison of normally distributed data (as for ‘age’). Mann–Whitney test was used whenever the normality assumption was not met (as for ‘follow-up time’). We used the Chi-square test to compare categorical data. We followed each patient through calendar date during the follow-up period, from the first CPI administration to the date TD was detected (i.e., time to TD), died (i.e., survival), or the last date of follow-up (October 2018). Mortality rates during the study period were estimated using the Kaplan–Meier method, and the differences between the groups were assessed by the log-rank test. A Cox proportional hazards regression analysis was performed to investigate the effect of selected variables on mortality during the follow-up period. All the clinical and demographic variables that differed significantly between groups by the univariate analysis were included in the first step of the model. The hazard proportionality for mortality was assessed by evaluating the interaction between log (survival time) and the variable of interest. Hazard ratios (HRs) and 95% confidence intervals were estimated for each variable in the final parsimonious models. All statistical analyses were performed with IBM SPSS Statistics, version 26. The threshold for statistical significance was set at a two-tailed p-value <0.05.
The study was approved by the institutional ethical board of Sheba Medical Center.
Results
Study cohort
We identified 934 patients treated with immune checkpoint inhibitors (CPIs) at the Chaim Sheba Medical Center Cancer Center during the study period, not enrolled in randomized placebo-controlled blinded trials. After excluding patients with no documented TFT, those treated with levothyroxine at baseline, and those with impaired TFT at baseline, 754 patients were included in the analyses. The study population included 299 (39.7%) women and 455 (60.3%) men. The patients’ mean age at CPI initiation was 61 ± 13 years. presents the characteristics of the patients who developed TD following CPI treatment and those who did not develop TD.
Table 1. Demographics, clinical characteristics in patients who developed thyroiditis versus those who did not develop thyroiditis.
Thyroid function dynamics
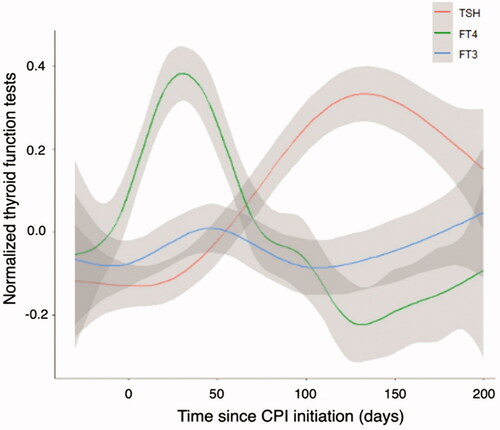
Thyroid function dynamics for the entire cohort, expressed as normalized values, are presented in , demonstrating a relatively large early increase in fT4, subsequent decrease in fT4 followed by an increase in TSH. Changes in fT3 were minor. Increased fT4 as the first indication of TD after CPI administration appeared earlier (mean of 110 ± 147 days; median 48.1 days) than low fT4 (163.9 ± 145.7 days; median 120.9 days) or high TSH.
Predisposing factors for TD
A total of 301 (39.9%) patients developed abnormal thyroid function following CPI treatment. 169 (22.4%) initially presented with high FT4 levels, 110 (36.5%) had high TSH, and 47 (6.2%) presented with low FT4 following CPI treatment. In addition, 26 patients (8.6%) without otherwise biochemical documentation of hypothyroidism in our hospital records were started on levothyroxine treatment. In 25 (8.3%) patients, both high and low FT4 levels were noticed at different time points during the follow-up. The risk for TD did not correlate with gender, BMI (body mass index) (), or tumor type. Stratification of the study population by age groups (<60, 60–70, >70, >75) revealed comparable rates of TD in all age strata (not shown). A comparable proportion of patients who developed TD was observed across tumor types: lung – 42.8% (n = 89), melanoma – 37.3% (n = 79), transitional cell carcinoma – 32.7% (n = 17), ‘other’ tumors – 39.2% (n = 100), all p > 0.05. However, a significantly higher rate of TD was observed in patients with renal cell carcinoma (59.3%; n = 16; p = 0.04).
The proportion of patients with TD according to the various therapeutic regimens are presented in . Of the PD-1 inhibitors, nivolumab was associated with a high rate for TD (43.8%) and 49% percent of patients who received ipilimumab in combination with nivolumab and patients treated with ipilimumab in any combination (49% in both groups). Conversely, the incidence of TD was particularly low in patients treated with durvalumab (22.9%).
Table 2. Thyroiditis rate, according to CPI treatment.
Survival rates in patients with thyroid dysfunction vs. patients with normal thyroid function
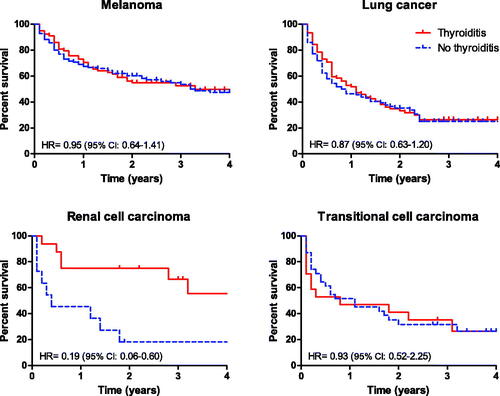
The Kaplan Meier survival analysis estimates for patients with and without CPI-induced TD are presented in . Overall, during the 5 years follow-up period, there was a non-significant trend toward improved survival in patients who developed TD in four predefined groups: melanoma, lung cancer, renal cell carcinoma, and transitional cell carcinoma. Nevertheless, we observed a highly significant survival benefit for patients with renal cell carcinoma who developed TD (HR = 0.19, 95% CI 0.06–0.60; p = 0.005).
Figure 2. Kaplan–Meier survival analysis in patients with and patients without thyroiditis, according to types of malignancies.
A Cox regression analysis for selected predictors of death is presented in . The Cox hazard ratio for death over the 5-year follow-up period was not affected by older age or gender. Patients treated with atezolizumab, pembrolizumab, and nivolumab had an increased risk for mortality; however, patients who developed thyroiditis and those treated with durvalumab seemed to be protected.
Table 3. Cox regression analysis for selected predictors of death.
Discussion
To the best of our knowledge, this is one of the largest studies assessing CPI-induced thyroid dysfunction from a single center. Our large patient cohort allowed for a robust assessment of the predispositions for developing TD while controlling for key covariates, including CPI types and combinations, age, gender, and tumor type.
Risk for developing thyroiditis
Compared with single-agent regimens, treatment with the combination of CPI therapy with ipilimumab and nivolumab, or any CTLA4 + PD1/PD-L1 inhibitors combinations, increased the likelihood of developing TD, emphasizing the synergistic effect of combined PD-1 and CTLA-4 blockade in terms of toxicity. Within the PD-1 inhibitors class, nivolumab alone was associated with a non-significant trend for a higher likelihood of developing TD than pembrolizumab. In contrast, treatment with durvalumab was associated with a significantly decreased likelihood of developing TD. These results generally correspond with previous observations on CPI-induced TD [Citation3,Citation13,Citation17].
Of interest, further analysis revealed that the likelihood to develop TD was increased in patients with renal cell carcinoma (59% vs. 39.9% overall; p = 0.04). The reason for this is not apparent, but it could be related to previous subliminal thyroid damage in patients previously treated with sunitinib, which is occasionally associated with clinically significant thyroid dysfunction [Citation18,Citation19]. This possibility is supported by observations indicating an increased rate of TD in patients with underlying thyroid disease, with preexisting anti-thyroid antibodies [Citation9,Citation15,Citation20–22], or with increased baseline TSH level [Citation23].
Thyroiditis and overall survival
The association of irAEs following CPI treatment with improved survival is still unsettled, with several studies suggesting a survival benefit and enhanced response with irAEs [Citation10–12,Citation14,Citation21,Citation24–28] and not in others [Citation13,Citation29]. Overall, we noticed only a marginal trend toward improved survival in patients who developed TD following CPI. However, we observed a significantly improved survival rate in patients with renal cell carcinoma who developed TD (). The reason for this remarkable association is not yet apparent. It could reflect the alleged increased susceptibility of renal cell carcinoma to immune manipulations [Citation30], rendering it exceptionally responsive to immunotherapy. On the other hand, it could suggest a possible tumor-specific interaction with the host, which simultaneously increases susceptibility toward CPIs-induced immune response both in the tumor and in other tissues, an association that deserves further study.
Thyroiditis and its clinical manifestations
The early increase of fT4 during the thyrotoxic phase concurrent with a lower increase in fT3 abnormalities () [Citation4] is compatible with the destructive nature of the mechanism of thyroid dysfunction (i.e., thyroiditis) [Citation5–7].
Concurrent with the rapidly expanding use of CPIs in cancer treatment, CPI-mediated TD is becoming more prevalent among cancer patients. There are several distinct characteristics of TD worth emphasizing when comparing it with thyroidal injuries induced by other therapeutic agents, for example, amiodarone, interferon alfa, interleukin-2, and tyrosine kinase inhibitors [Citation19]. The early onset of CPI-induced TD and its relatively rapid progression are distinctive and conceivably reflect the prompt and extensive recruitment of cytotoxic T-cells and the particular vulnerability of the thyroid gland to autoimmune attack [Citation5–7,Citation31].
Despite rare reports on severe thyrotoxicosis and even ‘thyroid storm’ in CPIs-treated patients [Citation32], the typical presentation of CPI-induced TD is exceptionally clinically indolent. The mild clinical presentation of the thyrotoxic phase can be explained by concurrent alterations in thyroid hormone metabolism and peripheral response to thyroid hormones during the CPI-induced intense immune reaction, characteristic of the ‘euthyroid-sick syndrome’, which often accompanies inflammatory and no-inflammatory conditions [Citation33]. Alterations in thyroid hormones and activity due to ‘euthyroid-sick syndrome’ include decreased expression of the thyroid hormone cell transporters MCT8 and MCT10, responsible for T4 uptake by peripheral tissues. Alterations in deiodinase expression result in reduced production of T3, simultaneous increased production of the biologically inactive metabolite, reverse-T3, and possibly a decrease in thyroid hormone receptors expression and their nuclear binding to DNA resulting in ‘tissue hypothyroidism’ [Citation33]. Due to its mild clinical presentation, unless there are clinical awareness and specific laboratory surveillance, the early phase of thyroiditis can be easily missed. Levothyroxine should be initiated for patients with hypothyroidism and immunotherapy should not be interrupted. Finally, whereas most other drug-induced thyroid abnormalities result in the recovery of normal thyroid function, with only 5–20% of cases resulting in permanent hypothyroidism [Citation19], most patients with CPI-induced TD progress to overt hypothyroidism requiring permanent treatment with a full replacement dose of thyroxine [Citation17].
Graves’ disease rarely occurs in the context of CPI treatment [Citation17] but should be considered, particularly, in severely symptomatic or protracted thyrotoxicosis. In rare cases with severe thyrotoxic manifestations, immunotherapy should be withheld and treatment with high-dose glucocorticoids initiated [Citation34].
Limitations
Our study’s limitations include its retrospective methodology, lack of data on non-CPI treatments before and after CPI administration that could be pertinent to survival. Data on anti-thyroid antibodies before CPI treatment was not systematically available since TFTs were often performed, and levothyroxine treatment was initiated by the family physicians. Nevertheless, data on CPIs treatments were complete. We believe that having defined CPI-induced TD according to either abnormal TFTs or initiation of levothyroxine therapy enabled us to capture most of the affected patients.
Conclusion
Our results, based on a large single-center cohort of patients with immune-related, checkpoint inhibitors-induced thyroid dysfunction, underlines the overall high likelihood of TD in CPI-treated patients, the even higher likelihood for TD in patients receiving combinations of CPI drugs and those treated for renal cell carcinoma; the lack of particular personal predisposing conditions, such as gender, age, or BMI [Citation35]; the early onset, but clinically indolent, course of TD, and the implicit -direct or indirect- impact of TD on patients’ survival, particularly those with renal-cell carcinoma. Further studies should address the biochemical-clinical discrepancy observed in most patients with TD, assess a possible role for developing de-novo anti-thyroid antibodies in the pathogenesis of TD [Citation22], and further explore the prognostic impact of TD on the survival of immunotherapy-treated patients with various malignancies.
Supplemental Material
Download MS Word (25.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s). Dr. Urban reports personal fees and non-financial support from Bristol Myers Squibb, personal fees from Merck Sharp and Dohme Israel, personal fees from ROCHE, personal fees and non-financial support from Astrazeneca, personal fees and non-financial support from Takeda, personal fees from Boehringer Ingleheim, outside the submitted work. Dr. Bar reports grant support for the institute and personal fees from Roche, Merck Sharp and Dohme Israel, Astrazeneca, Abbvie, Pfizer, Novartis and Takeda, and personal fees from Boehringer Ingelheim and Bayer, outside the submitted work.
References
- Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29(1):71–83.
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168.
- Chang LS, Barroso-Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17–65.
- de Filette J, Jansen Y, Schreuer M, et al. Incidence of thyroid-related adverse events in melanoma patients treated with Pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439.
- Kotwal A, Gustafson MP, Bornschlegl S, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. 2020;30(10):1440–1450.
- Angell TE, Min L, Wieczorek TJ, et al. Unique cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes Dis. 2018;5(1):46–48.
- Imblum BA, Baloch ZW, Fraker D, et al. Pembrolizumab-induced thyroiditis. Endocr Pathol. 2019;30(2):163–167.
- Kobayashi T, Iwama S, Yasuda Y, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc. 2018;2(3):241–251.
- Mazarico I, Capel I, Giménez-Palop O, et al. Low frequency of positive antithyroid antibodies is observed in patients with thyroid dysfunction related to immune check point inhibitors. J Endocrinol Invest. 2019;42(12):1443–1450.
- Peiró I, Palmero R, Iglesias P, et al. Thyroid dysfunction induced by nivolumab: searching for disease patterns and outcomes. Endocrine. 2019;64(3):605–613.
- Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30(2):177–184.
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or Placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6(4):519–527.
- Al Mushref M, Guido PA, Collichio FA, et al. Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract. 2020;26(1):36–42.
- Akamatsu H, Murakami E, Oyanagi J, et al. Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncologist. 2020;25:e679.
- Basak EA, van der Meer JWM, Hurkmans DP, et al. Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30(7):966–973.
- Centanni M, Moes DJAR, Trocóniz IF, et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58(7):835–857.
- Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28(10):1243–1251.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590.
- Burch HB. Drug effects on the thyroid. N Engl J Med. 2019;381(8):749–761.
- Alhusseini M, Samantray J. Hypothyroidism in cancer patients on immune checkpoint inhibitors with anti-PD1 agents: insights on underlying mechanisms. Exp Clin Endocrinol Diabetes. 2017;125(4):267–269.
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589.
- de Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. 2019;7(1):6–11.
- Pollack RM, Kagan M, Lotem M, et al. Baseline TSH level is associated with risk of anti-PD-1-induced thyroid dysfunction. Endocr Pract. 2019;25(8):824–829.
- Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–1365.
- Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378.
- Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730–2737.
- Aso M, Toi Y, Sugisaka J, et al. Association between skin reaction and clinical benefit in patients treated with anti-programmed cell death 1 monotherapy for advanced non-small cell lung cancer. Oncologist. 2020;25:e536.
- Kichenadasse G, Miners JO, Mangoni AA, et al. Multiorgan immune-related adverse events during treatment with atezolizumab. J Natl Compr Canc Netw. 2020;18(9):1191–1199.
- Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198.
- Ross K, Jones RJ. Immune checkpoint inhibitors in renal cell carcinoma. Clin Sci. 2017;131(21):2627–2642.
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–265.
- Yonezaki K, Kobayashi T, Imachi H, et al. Combination therapy of ipilimumab and nivolumab induced thyroid storm in a patient with Hashimoto’s disease and diabetes mellitus: a case report. J Med Case Rep. 2018;12:171.
- Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest. 2021;44(8):1597–1607.
- Del Rivero J, Cordes LM, Klubo-Gwiezdzinska J, et al. Endocrine-related adverse events related to immune checkpoint inhibitors: proposed algorithms for management. Oncologist. 2020;25(4):290–300.
- Pollack R, Ashash A, Cahn A, et al. Immune checkpoint inhibitor-induced thyroid dysfunction is associated with higher body mass index. J Clin Endocrinol Metab. 2020;105(10):e3620–e3627.