?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Only few existing studies have investigated the mortality from cardiovascular disease (CVD) in women with breast cancer (BC). The aim of this study was to investigate CVD mortality in patients with BC compared with a matched control group without BC using national registry data.
Material and methods
We followed 16,505 Danish women diagnosed with BC in 2003–2007 up to 10 years after BC diagnosis compared with 165,042 matched controls from the general Danish population. The matching criteria included gender, age, region of residence, and education. We performed multivariate Cox regression analyses to investigate the influence of preexisting CVD on mortality. Moreover, we used the cumulative incidence and conditional probability functions to study the risk of CVD-related death in the presence of competing risk, i.e., the risk of dying from other causes than CVD.
Results
We found that preexisting CVD increased both overall mortality and CVD mortality in both patients with BC and controls. Furthermore, we found that patients with BC were at lower risk of dying from CVD up to 10 years after BC diagnosis compared with controls. The cumulative incidence of CVD as underlying cause of death was 4.0% in patients with BC and 5.7% in controls after 10 years. The most common CVD-related causes of death were ischemic heart disease including acute coronary syndrome, cerebrovascular accident, heart failure, and atrial fibrillation.
Discussion
Our study contributes to the growing body of work on BC and comorbidities and highlights the importance of CVD in individuals with BC. Further studies are needed to confirm our finding that patients with BC are at lower risk of dying from CVD up to 10 years after BC diagnosis compared with a matched control group without BC
Background
Breast cancer (BC) is the most frequent type of cancer in women worldwide and responsible for approximately 25% of all new cancer cases in women [Citation1]. In Denmark as in other Northern European countries, BC incidence has increased over recent decades [Citation2]. In combination with improved survival rates [Citation2], this has resulted in a growing number of BC survivors; the majority of whom will die from other causes than BC.
The risk of dying from cardiovascular disease (CVD) may be higher in BC survivors due to cardiotoxic effects of some cancer treatments as well as shared risk factors between BC and CVD [Citation3,Citation4]. For example, metabolic syndrome (cluster of interrelated abnormalities including central obesity, insulin resistance, dyslipidemia, and hypertension) is a well-known risk factor of CVD and has also been associated with increased of risk of BC [Citation5]. It is widely documented that comorbidity, including CVD, is associated with higher mortality in patients with BC [Citation6–11]. It is, however, still unclear whether CVD mortality is higher in BC survivors compared with women without BC. Most existing studies have investigated CVD mortality in BC survivors without comparison to the general population. A recent literature review [Citation3] found only two studies investigating CVD mortality in BC survivors compared with women without BC [Citation12,Citation13].
The aim of this study was to investigate CVD mortality in patients with BC compared with a matched control group without BC based on a large real-world dataset covering the entire Danish population. Compared with previous studies [Citation12,Citation13], our study includes a larger and less segmented study population of patients with BC and a more precisely matched control group.
Material and methods
In this population-based cohort study, we studied CVD mortality in patients with BC up to 10 years after BC diagnosis compared with a matched control group without BC.
Study population
The group of patients with BC consisted of all women 18+ years old diagnosed with BC in Denmark between 1 January 2003 and 31 December 2007 without prior cancer diagnosis. We used the International Classification of Disease 10th revision (ICD10) code C50* to identify patients with BC, date of diagnosis and cancer disease stage when diagnosed (with and without distant metastasis). We retrieved this information from the Danish Cancer Registry. The Danish Cancer Registry is a research registry containing information on the incidence of cancer in the Danish population since 1943 [Citation14]. We used the diagnosis date as index date. As we focused on newly diagnosed cancer-naïve patients with BC, we excluded women with any cancer diagnosis prior to the index date. Due to lack of data on patients diagnosed with cancer outside Denmark, we also excluded women who had lived outside Denmark for more than one year when they were 18+ years old.
We used exact matching to identify a control group of women from the general population in Denmark. First, we assigned a random index date to each 18+ year old woman without BC in Denmark (the potential control group). In the same manner as the BC group, we excluded women with any cancer diagnosis up to and including the index year as well as women who had lived outside Denmark for more than one year when they were 18+ years old. Then, we selected 10 controls for each BC patient matched according to index year, age (<30, 30–34, 35–39, …, 85+), region of residence (Capital, Zealand, Southern Denmark, Central Jutland, or Northern Jutland) and education (low, medium, high, or unknown).
Outcome
Our primary outcome of interest was CVD-related death. We relied on information from the Danish Register of Causes of Death [Citation15] and distinguished between different types of CVD-related deaths based on the following ICD10 codes:
Heart valve disease: I050, I051, I340, I341, I342, I349, I350, I351, I359;
Hypertension: I10, I11, I12, I13, I14, I15, I110, I130, I132);
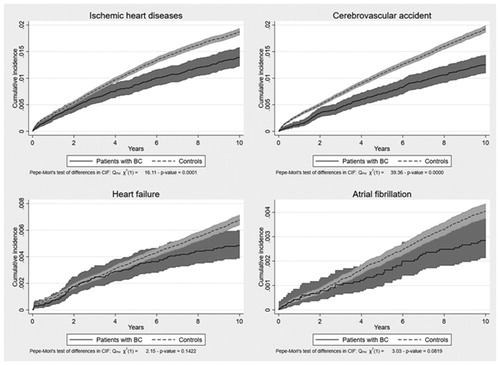
Ischemic heart diseases and acute coronary syndrome: I20, I21, I22, I25, I252;
Pulmonary heart disease: I26, I27, I28;
Atrial fibrillation: I48;
Other cardiac fibrillations: I49;
Heart failure: I420, I426, I427, I429, I110, I130, I132, I50;
Peripheral vascular disease: I790, I739, Z958, Z959, I71, R02;
Thrombophlebitis and thrombosis: I80, I82;
Cerebrovascular accident: I60, I66, G46, I69, I670, I672, I674, I679, I681, I682, I688, G451, G452, G454, G458, G459.
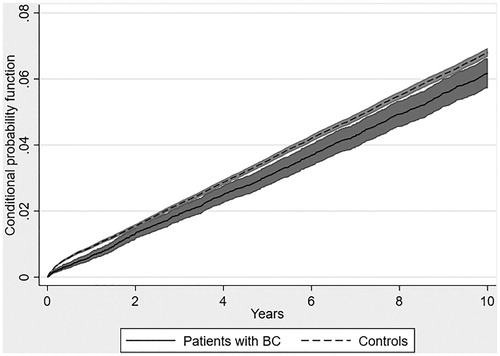
In the main analysis, we included CVD-related deaths reported as underlying cause of death, i.e., ‘the disease or injury, which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury’ [Citation16]. We also performed a sensitivity analysis where we included CVD-related deaths reported as either underlying, direct or contributing cause.
Covariates
We defined CVD at index date as at least one primary or secondary CVD diagnosis in the National Patient Registry up to five years prior to the index date. The National Patient Registry contains information on all patients discharged from Danish hospitals since 1977 and on emergency department and outpatient visits since 1995 [Citation17]. For each hospital contact, the registry holds information on one primary and optional secondary diagnoses, where the primary diagnosis is the main reason for the hospital contact and secondary diagnoses identify other relevant diseases.
Similarly, we defined diabetes at index date as at least one primary or secondary diabetes diagnosis in the National Patient Registry up to five years prior to the index date.
Data on age, residence, and education were obtained from the Civil Registration System [Citation18] and national education registers [Citation19].
Statistical analysis
First, we performed multivariate Cox regression analyses to investigate the influence of CVD at index date on all-cause mortality (model 1) and CVD mortality (model 2) during the 10-year follow-up period. Patients with BC and controls were analyzed separately with persons without CVD at index date as reference.
Next, we used the cumulative incidence function to study the risk of CVD-related death in patients with BC and controls as other recent studies [Citation13,Citation20,Citation21]. The cumulative incidence function allows for estimation of the incidence of the occurrence of an event such as CVD-related death taking competing risks into account [Citation22]. A competing risk is an event whose occurrence precludes the occurrence of the primary event of interest [Citation22,Citation23]. For example, if a person dies from BC, this person cannot die from CVD. Common practice is to consider a competing risk as a censoring event and use traditional estimators from survival analysis when computing the risk of the primary event of interest. However, the complement of the Kaplan–Meier estimator of the survival function, also known as the failure function, will overestimate this risk [Citation24]. In the presence of competing risks, we need to take into account that the other causes of death indeed influence the probability of surviving, and that individuals who have died from other causes are no longer at risk of dying from the cause under study [Citation25]. We followed patients with BC and controls from the index date until the occurrence of one of the following three events: death, emigration, or end of the 10-year follow-up period (whatever happened first). Our primary event of interest was CVD-related death, and we treated all other causes of deaths as competing risks. We applied the cumulative incidence formula by Marubini and Valsecchi [Citation26] and calculated 95% confidence intervals by log transformation ( where
is the cumulative incidence function) [Citation27].
The cumulative incidence function does not offer complete understanding of competing risk data, as the cumulative incidence function of the event of interest may appear low only because the cumulative incidence function of the competing risk is high [Citation26]. Therefore, we also computed the conditional probability function, which shows the probability at time t of observing the event of interest conditional on the individual not experiencing a competing risk event at time t (mathematically, it is the failure function of the primary event of interest divided by the complement of the failure function for the competing risk events).
The analyses were performed using Stata 14.2 (StataCorp, College Station, TX, USA). We evaluated the statistical significance of differences between groups using 95% confidence intervals as well as two-sided t-tests and Pepe and Mori’s tests with 5% level of significance. The Pepe and Mori’s test was used when comparing the cumulative incidence functions of patients with BC and controls [Citation28,Citation29].
Results
The study population included 16,505 patients with BC and 165,042 matched controls (see ). In a previous study, we found that CVD and diabetes comorbidity at index date were slightly higher in patients with BC than controls [Citation30].
Table 1. Baseline characteristics of patients with BC and controls.
At the end of the 10-year follow-up period, 39% of patients with BC and 21% of controls had died (data not shown). Mortality from all causes as well as CVD mortality were higher in persons with CVD at index date compared with persons without CVD (see ). However, the hazard ratio (HR) of dying of all causes with CVD at index date was higher in controls (HR = 1.8) than in patients with BC (HR = 1.4) (see , model 1) which may be explained by higher mortality in patients with BC in general. When focusing on deaths from CVD with CVD at index date, HR was similar in patients with BC (HR = 2.5) and controls (HR = 2.6) (see , model 2).
Table 2. Multivariable analysis of hazard ratio (HR) of death with CVD at index date (persons without CVD at index date as reference).
The cumulative incidence function showed a lower risk of dying from CVD in patients with BC compared with controls during the 10-year follow-up, including both persons with and without CVD at index date (see ). The conditional probability function confirmed this picture (see ). The risk of dying from CVD as underlying cause of death was 4.0% in patients with BC and 5.7% in controls after 10 years (see ). The risk of dying from CVD remained lower in patients with BC than controls in the sensitivity analysis where all CVD-related deaths were included no matter whether reported as underlying, direct or contributing cause (see ). However, the difference between patients with BC and controls decreased compared with the main analysis.
Figure 1. Cumulative incidence of CVD-related death in the presence of competing risks, proportion with CVD-related death.
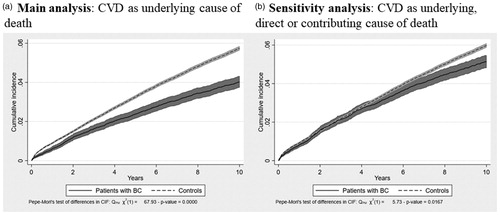
Figure 2. Conditional probability of CVD-related death, proportion with CVD-related death conditional on the individual not experiencing a competing risk event.
The most common CVD-related causes of death in the main analysis were ischemic heart disease including acute coronary syndrome, cerebrovascular accident, heart failure, and atrial fibrillation. The cumulative incidence of death from all four types of CVD was higher in controls than in patients with BC within the 10-year follow-up period (see ).
Discussion
We used registry data covering the entire Danish population to investigate CVD mortality in patients with BC up to 10 years after BC diagnosis compared with a matched control group without BC. We found that CVD at index date increased CVD mortality in both BC survivors and controls. This finding is in accordance with existing evidence as numerous studies have shown that patients with BC with comorbidity are at increased risk of dying of BC as well as other causes [Citation6–11]. By focusing on CVD mortality in particular, our study contributes to existing evidence in this area.
As our main contribution, we found that patients with BC were at lower risk of dying from CVD up to 10 years after BC diagnosis compared with controls in the presence of competing risks. This included deaths from CVD in general as well as deaths from specific types of CVD such as ischemic heart disease including acute coronary syndrome, cerebrovascular accident, heart failure, and atrial fibrillation. This finding is interesting as a literature review from 2017 on different aspects relating to CVD in patients with BC [Citation3] found that CVD mortality was higher in women with BC compared with women without BC. This conclusion is mainly based on existing studies by Bradshaw et al. and Riihimaki et al. [Citation12,Citation13].
The study by Bradshaw et al. [Citation12] included 1413 American patients with BC diagnosed in 1996–1997 and compared them with 1411 age-matched controls without BC taking the presence of competing risks into account. Vital status and primary causes of death until 2009 were established based on registry data. Bradshaw et al. found that the risk of CVD-related death was higher among in patients with BC than in the matched control group without BC, but the increased risk was evident only seven years after BC diagnosis. In the early years after BC diagnosis, the risk of CVD death was lower in patients with BC compared with controls. The study by Bradshaw et al. included a 10 times smaller study population than in our study, and the patients included were invited to participate which may have resulted in a more selected group of patients with BC with relatively good prognosis.
The study by Riihimaki et al. [Citation13] investigated the risk of dying from different causes including CVD in Swedish patients with BC compared with a control group without BC. The study was based on registry data covering all women born between 1931 and 1977 and residing in Sweden during the study period 1987–2006. Riihimaki et al. found that patients with BC had increased risk of dying of various causes, including heart failure and diseases in pulmonary circulation, compared with the control group. Riihimaki et al. relied only on traditional Cox regression models and did not compute cumulative incidence or conditional probability functions to analyze the risk of CVD-related death in the presence of competing risks, as we did in our study. As presented in section ‘Material and Methods’, the risk of dying from CVD may be overestimated when competing risks are not taken into account.
A recent nationwide registry study by Buddeke et al. [Citation21], not included in the literature review from 2017 mentioned above [Citation3], investigated CVD mortality in 163,881 Dutch women admitted to hospital for BC during 1996–2010 compared with women from the general population. They found that the risk of dying from CVD was lower in patients with BC than in women from the general population, as we did. The 10-year CVD mortality decreased from 5.6% in patients with BC diagnosed in 1996 to 4.1% in patients with BC diagnosed in 2005 (relative reduction of 26.8%), and from 7.3% to 5.5% in women from the general population in the same period (relative reduction of 24.7%). There is thus a striking similarity between our results and the 10-year CVD mortality estimated by Buddeke et al. for the 2005 population.
A new study by Ramin et al. investigated CVD mortality in 628 American women diagnosed with early stage BC over a follow-up period up to 25 years [Citation20]. They find a higher CVD mortality for patients with BC after 10 years compared to a matched control group but only for women diagnosed before 2005.
Both the study by Buddeke et al. as well as the study by Riihimaki et al. compared women with BC with women from the general population adjusting for differences in age. Bradshaw et al. and Ramin et al. also included a geographical parameter by identifying a control group consisting of women without BC from the same area as patients with BC matched 1:1 and 1:5 respectively according to age. In our study, though, we matched 1:10 according to both gender, age, region of residence, and education in order to make the group of patients with BC and controls more comparable.
Differing results may also reflect changes in CVD mortality in patients with BC over time following efforts to reduce the risk of CVD induced by BC treatments as well as improvements in pharmacological prevention of CVD and non-pharmacological prevention programs. It is possible that increased focus on cardio-oncology and closer cardiac monitoring of patients with BC after BC diagnosis today results in earlier diagnosis and better treatment of CVD compared to the general population. The higher focus on cardio-oncology today could help to improve this. Furthermore, positive lifestyle changes, which reduce the risk of CVD-related death, may be more prevalent among patients with BC after BC diagnosis following recommendations to improve BC prognosis and optimize overall health [Citation31].
Strengths and limitations
The present study is a study at the population level including women diagnosed with BC in Denmark during the period 2003–2007 as well as a control group without BC matched to patients with BC according to gender, age, region of residence, and education. Women with BC were identified from the Danish Cancer Registry, which has a high completeness and validity [Citation14], and we used the cumulative incidence and conditional probability functions to analyze CVD mortality in the presence of competing risks [Citation22,Citation23]. Since patients with BC had a higher risk of dying during the 10-year follow-up period than controls, ignoring competing risks could induce a substantial bias. Our study design allowed us to compare CVD mortality in women with BC and women without BC and produce results that are applicable at the national level. These are the major strengths of our study.
Our study also has limitations. The study relied on death certificate information in the Danish Register of Causes of Death, which is a potential source of misclassification. A recent study from Norway [Citation32] assesses the validity of cause of death when attributed to cancer and conclude that for most cancers including BC the cause of death attributed to cancer is correctly reported. Hence, the risk of misclassification of cause of death due to BC should be low. The results from Norway are likely transferable to Denmark. In our study, less than 10% of patients with BC, who are registered with BC as underlying cause of death, had no contact to hospital with a cancer diagnosis within 6 months before time of death.
We have not found Danish studies that examine the validity of death causes for BC patients, but a recent Danish study [Citation33] has examined the validity of death certificates for prostate cancer patients in the Danish Register of Causes of Death against blinded medical records review. This study found that information in the Danish Register of Causes of Death overestimate the proportion of deaths attributable to prostate cancer, the proportion of misattribution ranging from 5% to 21%. However, the survival rate for prostate cancer is high and these patients tend to die at an old age where cause of death is more difficult to assess.
The share of patients with BC who die of BC in our study declines with age of death, whereas the share of people dying of CVD increases with age for both groups. Survival time seems unrelated to age of death. If physicians tend to attribute the cause of death to cancer for patients with cancer, we expect this tendency to diminish with time since BC diagnosis resulting in an increase in the share dying from other causes, including CVD. In the group of patients with BC, who die during the 10-year follow-up period, the share of women who are registered as dying of BC is falling over time, whereas the share who is registered as dying of CVD is constant over time and around 10%. This indicates that misclassification in the Register of Causes of Death is probably not a major problem in our study.
Another limitation of our study is our relatively short 10-year follow-up period compared to the study by Ramin et al. Further research with longer follow-up might show a higher CVD mortality for patients with BC.
Furthermore, there is always a risk of confounding in observational studies. The risk of confounding related to age, region of residence, and education was minimized by exact matching in our study. Furthermore, our data showed that patients with BC and controls were almost similar with regard to CVD and diabetes comorbidity at index date. Still, patients with BC and controls in our study might differ on non-observable factors such as smoking and other lifestyle factors, which influence CVD mortality. However, we believe that the risk of confounding due to such non-observable factors was limited because our study was carried out at the population level, including all women diagnosed with BC in Denmark in 2003–2007 without a prior cancer diagnosis. Furthermore, patients with BC and controls were matched according to education, which is often used as a proxy for lifestyle.
Conclusion
In summary, our study results show that preexisting CVD increase CVD mortality in both patients with BC and controls during a 10-year follow-up period. Our main finding is a lower CVD mortality in patients with BC compared with controls, including both persons with and without CVD at index date. This result may reflect closer cardiac monitoring and increased focus on cardio-oncology, resulting in earlier diagnosis and better treatment of CVD in patients with BC. Further studies are needed to confirm that patients with BC have a lower risk of dying from CVD up to 10 years after BC diagnosis and identify possible reasons.
Disclosure statement
CK, MJ, and MSJ are employees of the National Center for Social Science Research (VIVE), which was a paid vendor to Pfizer Denmark on the project. VIVE is an independent research institute, which is under obligation by law to disseminate the results of its work to relevant public and private stakeholders and the public in general. MA and TK are medical doctors with expertise in breast cancer and cardiovascular disease, respectively, and both report personal fees from Pfizer Denmark during the conduct of the study. PBP and HK are employees of Pfizer Denmark and own shares in Pfizer Inc. outside submitted work.
Additional information
Funding
References
- The Global Cancer Observatory. World fact sheet; 2021; [Internet]. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- NORDCAN. NORDCAN projektet – Kraeftstatistik for de nordiske lande; 2021; [Internet]. Available from: http://www-dep.iarc.fr/NORDCAN/DK/frame.asp
- Gernaat SAM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–555.
- Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66.
- Schmoldt A, Benthe HF, Haberland G. Metabolic syndrome and the risk of breast cancer and subtypes by race, menopause and BMI. Biochem Pharmacol. 1975;24(17):1639–1641.
- Cronin-Fenton DP, Nørgaard M, Jacobsen J, et al. Comorbidity and survival of Danish breast cancer patients from 1995 to 2005. Br J Cancer. 2007;96(9):1462–1468.
- Ewertz M, Land LH, Dalton SO, et al. Influence of specific comorbidities on survival after early-stage breast cancer. Acta Oncol. 2018;57(1):129–134.
- Land LH, Dalton SO, Jensen MB, et al. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2012;131(3):1013–1020.
- Land LH, Dalton SO, Jensen MB, et al. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer. 2012;107(11):1901–1907.
- Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl. 1):3–29.
- Land LH, Dalton SO, Jorgensen TL, et al. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol. 2012;81(2):196–205.
- Bradshaw PT, Stevens J, Khankari N, et al. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13.
- Riihimaki M, Thomsen H, Brandt A, et al. Death causes in breast cancer patients. Ann Oncol. 2012;23(3):604–610.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl.):42–45.
- Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 Suppl.):26–29.
- World Health Organization (WHO). International statistical classification of diseases and related health problems, 10th revision. Vol. 2, instruction manual. 2010 ed. Geneva, Switzerland: WHO; 2011.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl.):30–33.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl.):22–25.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl.):91–94.
- Ramin C, Schaeffer ML, Zheng Z, et al. All-cause and cardiovascular disease mortality among breast cancer survivors in CLUE II, a long-standing community-based cohort. J Natl Cancer Inst. 2021;113(2):137–145.
- Buddeke J, Gernaat SAM, Bots ML, et al. Trends in the risk of cardiovascular disease in women with breast cancer in a Dutch Nationwide Cohort Study. BMJ Open. 2019;9(5):e022664.
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Clin Epidemiol. 2016;133(6):601–609.
- Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007;26(6):1360–1367.
- Pintilie M. Introduction; competing risks – definitions; descriptive methods for competing risks data. Competing Risks. 2006.
- Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706.
- Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester: Wiley; 1995.
- Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4(2):103–112.
- Pepe MS, Mori M. Kaplan–Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737–751.
- Coviello E. STPEPEMORI: Stata module to test the equality of cumulative incidences across two groups in the presence of competing risks, statistical software components, S456899. Boston College Department of Economics (revised 2010); 2008.
- Jakobsen M, Jensen M, Kolodziejczyk C. Komorbiditet ved brystkraeft – Et registerstudie om konkurrerende sygdom før og efter brystkraeftdiagnose med fokus på hjerte-kar-sygdom. Copenhagen: VIVE; 2020.
- Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ. 2017;189(7):E268–E274.
- Skyrud KD, Bray F, Møller B. A comparison of relative and cause-specific survival by cancer site, age and time since diagnosis. Int J Cancer. 2014;135(1):196–203.
- Nguyen-Nielsen M, Moller H, Tjonneland A, et al. Causes of death in men with prostate cancer: results from the Danish Prostate Cancer Registry (DAPROCAdata). Cancer Epidemiol. 2019;59:249–257.