Abstract
Background
Cancer-related fatigue is one of the most prevalent and distressing symptoms among cancer patients, resulting in a great cancer research challenge. Numerous systematic reviews of physical training interventions have been conducted to find the most effective approach. However, evidence remains fragmented, and in which cancer population physical training is more effective than other populations is still unclear. Thus, this study critically appraised systematic reviews and meta-analyses on physical training to reduce adults’ cancer-related fatigue.
Methods
A systematic review of systematic reviews and meta-analysis (PROSPERO: CRD42020189049), assessing the efficacy of exercise training for reducing cancer-related fatigue in adults, was conducted in PubMed, CINAHL, Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects, and Pedro. The selected studies (standardized mean difference, SMD; 95%CI), was quantitatively pooled using a random-effects model. Heterogeneity was tested using chi-squared (Q) and I-square statistics (I2).
Results
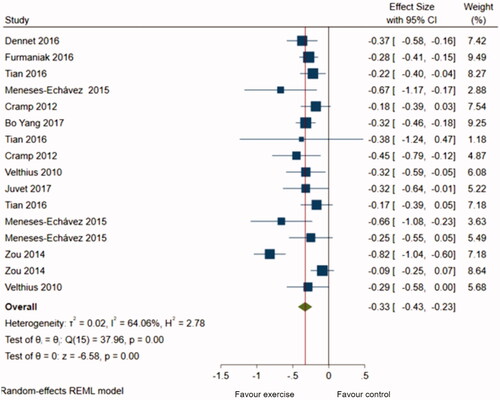
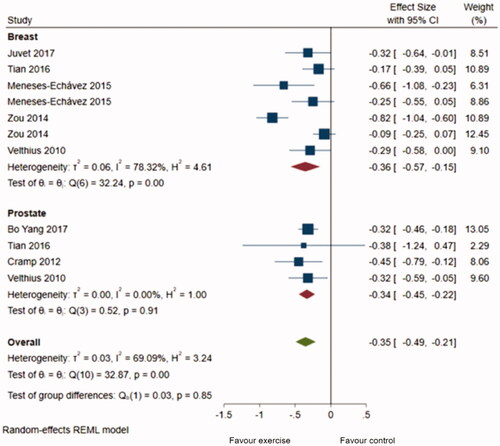
Of 1438 identified articles, 11 met the inclusion criteria, and ten were meta-analyzed. The results yielded a positive effect of physical training on fatigue in all cancer populations, SMD = −0.33 (−0.43, −0.23). Subgroup analysis based on tumor localization showed a slightly higher physical training effect on fatigue in adults with breast cancer, SMD = −0.36 (−0.57, −0.15), and prostate cancer SMD = −0.34 (−0.45, −.0.22).
Conclusions
Our analysis demonstrated some potential improvement in cancer-related fatigue in adult patients undergoing physical training during and after cancer treatments, particularly in patients with breast or prostate cancer.
Introduction
Cancer-related fatigue (CRF) is one of the most prevalent and distressing syndromes among cancer patients. The overall prevalence is roughly 49% varying across cancer populations, depending on tumor type (ranging from 14.03 to 100%), cancer stage (60.6% in advanced cancer), and treatment status (62% during treatment) [Citation1]. CRF represents a ‘driver’ of health issues of global health among cancer patients, considering its disruptive interaction with social and functional quality of life (QoL) domains [Citation2]. Given this interaction, fatigue may be a predictor of shorter survival [Citation3], considering that dose intensity or delay of some cancer treatments may be based on symptom severity [Citation4], and poor physical functional status is significantly associated with interruptions of treatments [Citation5].
Growing attention has been placed on the effects of physical activity in enhancing the psychological, physical, and emotional quality of life among cancer survivors during cancer treatment and survivorship as a part of routine cancer care [Citation6,Citation7]. Precisely, the benefit of physical activity on quality of life may be mediated by the positive effect of physical exercise on CRF, indicating an inversional proportional correlation of fatigue with quality of life [Citation8–10]. Furthermore, physical activity has been found to reduce cancer-specific mortality among breast, prostate, and colorectal survivors [Citation11–13]. Given these considerations, there is consistent evidence that exercise improves both aerobic capacity and muscular strength, neutralizing tumor and treatments’ negative effects on physical performance and reducing the perceived fatigue level [Citation14].
Nevertheless, cancer is a highly heterogeneous disease that evolves through numerous pathways involving differences in somatic alterations and symptom presentations within the same population cluster [Citation15]. This heterogeneity complicates identifying effective and appropriate interventions to foster the multidimensional nature of cancer symptoms, such as fatigue, which remains a great challenge and the highest priority research areas of the National Cancer Institute Clinical Oncology Research Program in the United States [Citation16]. In practice, different fatigue dimensions, such as physical and mental, differ in how they behave, demonstrating variable responses to interventions [Citation17].
In the last decades, numerous efforts have been conducted to identify the most effective approach, resulting in several randomized controlled trials on cancer-related fatigue management. As a result, literature encompasses multiple systematic reviews and meta-analyses on the efficacy of physical exercise for improving cancer-related fatigue. However, understanding whether physical exercise in a specific cancer population could be more effective than other cancer populations is still unclear, and knowledge remains fragmented. For this reason, providing a clear understanding of the efficacy of physical exercise on specific cancer populations in terms of tumor type could be strategic for addressing tailored and targeted interventions and avoid healthcare wastes. Thus far, this study aims to systematically review the available evidence about physical exercise’s effects on improving adult cancer patients' fatigue. In particular, we aim to identify which cancer population can mostly benefit from physical exercise interventions for reducing cancer-related fatigue. This knowledge could be used to optimize cancer care interventions.
Material and methods
This study is a systematic review of systematic reviews consistent with the suggested stepwise approach [Citation18]. The rationale is based on a preliminary literature search on non-pharmacological intervention to reduce cancer-related fatigue, which uncovered numerous systematic reviews and metanalysis on physical exercise for improving fatigue in cancer patients. The review protocol was firstly registered on PROSPERO (CRD42020189049) [Citation19,Citation20].
Search strategy and sources
Multiple databases were searched between January 2010 and August 2020, and re-checked in June 2021: PubMed, CINAHL, Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects and Pedro. We adopted a purposive sampling limiting the search to cancer subset and systematic reviews, abstract available, selecting methods-based filters (PubMed Clinical Queries) and topic-specific filters (Topic-Specific/Special Queries) [Citation21,Citation22]. According to recent literature, we combined MESH words and free-text words using a single-line search strategy [Citation23]. Foremost, the search strategy was designed for the PubMed database and then fixed for the other databases as appropriate (Supplementary File 1). The research was not limited to any language initially to find all the relevant publications. The reference list was cross-referenced and hand-searched to identify any additional articles [Citation24]. The last search was re-run before the final analysis in January 2021.
Eligibility criteria
This systematic review is based on Population, Intervention, Control, Outcomes, and Study design (PICOS) framework: (P) Adult cancer patients (≥18 years), (I) Aerobic, anaerobic and physical resistance exercise, (C) Usual care or no intervention or any type of intervention, (O) cancer-related fatigue as a primary endpoint, (S) systematic reviews and meta-analysis which include randomized controlled trials (RCTs) or longitudinal RCTs. Systematic reviews including patients with malignant hematological diseases were excluded, considering this population cluster [Citation25]. No restrictions on sex and ethnicity were settled as they were not significant for the study’s purposes. Yoga interventions were excluded because they pertain to Chinese medicine approaches. Relaxing or stretching exercises were not considered as interventions. Systematic reviews that comprised uncontrolled trials, case studies, studies without a control group, pilot, feasibility studies, and theoretical approaches were excluded.
We examined studies published in English in peer-reviewed journals (or accepted for publication), containing an abstract, and published between 2010 and May 2021 to identify the most relevant up-to-date international evidence on this topic.
Data selection
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and flowchart to guarantee a systematic selection process [Citation26]. Two authors (SB and RC) independently screened titles and abstracts to select pertinent studies for full-text review and final inclusion, consistently with the inclusion criteria. A consensus discussion solved disagreements in the inclusion process of abstracts and full-text articles. The authors discussed inclusion or exclusion reasons to ensure that the screening process was reliable and consistent.
Quality appraisal
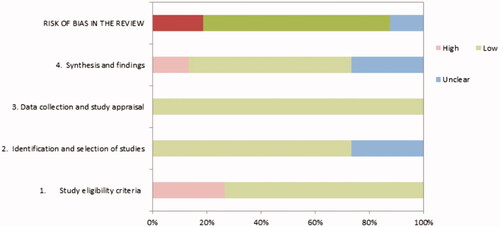
Two authors (SB and RC) independently evaluated the methodological quality of each systematic review selected at the final stage, using the ROBIS checklist [Citation27], according to recent Cochrane group suggestions for review of systematic reviews study design [Citation28,Citation29]. We graded each risk of bias as high, low, and unclear based on the ROBIS tool guidance recommendations [Citation30]. The ROBIS tool consisted of three phases: (1) relevance assessment, (2) review process evaluation, and (3) judgment of overall risk of bias. Specifically, phase two required assessing four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. Only systematic reviews of low risk of bias were included to restrict reporting of high-quality publications. A consensus discussion solved disagreements between the two authors in the quality rating process.
Data extraction and synthesis
The authors examined the selected systematic reviews several times to get an in-depth overview of the contents [Citation31]. Two authors (SB and RC) independently extracted and entered the data from studies into a standard paper extraction form, firstly tested throughout a piloting process. A consensus discussion resolved disagreements among the two authors. The following data were extracted from studies that encountered the selection criteria for inclusion: first author/year, number of studies included in the systematic review, number of participants, type of cancer, intervention, comparison, phase of treatment, CRF measurements, and results. Authors were contacted if the reported data were insufficient or unclear. We did not consider results from subgroup analysis with less than three studies during data extraction and limited simple size during data extraction. Results are presented according to the Cochrane Handbook of Systematic Reviews of Interventions [Citation28] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [Citation26].
Statistical analysis
We quantitatively pooled the effect size (standardized mean difference, SMD; 95% CI) to estimate the magnitude of physical activity’s effect in reducing cancer-related fatigue (from positive to negative). The magnitude of the SMD was considered as follows: small, SMD = 0.2; medium, SMD = 0.5; and large, SMD = 0.8 was considered [Citation32]. Given the high clinical heterogeneity between studies, a random-effects model of the inverse variance was used to calculate the pooled effect [Citation32], applying the restricted maximum likelihood (REML) [Citation33]. The magnitude of inconsistency between studies was estimated by chi-squared (Q) and I-square statistics (I2) [Citation34]. Considering that the performed model adopted the effect sizes of previously published meta-analysis to obtain a pooled estimate, the possible overlapping of primary studies in the model has been manually checked. In the eleven included systematic reviews (only 10 systematic reviews with meta-analyses), only 11 primary studies of 149 were included twice.
For this reason, we empirically tested whether excluding the information of the 11 overlapping primary studies significantly changed the pooled effect size of the model by performing a model from the effect sizes of the primary studies. The overall SMDs in both models (one model was a meta-analysis of meta-analyses, the other was a meta-analysis including the primary studies without duplicating the information of the 11 overlapping studies) were not significantly different. Then, we empirically deduced that the overlapping effects of primary published studies were marginally relevant in defining the effect size of the meta-analysis of meta-analyses. Considering that the aim of a systematic review of systematic reviews is about providing an understandable synthesis of the available literature reviews rather than exploring new associations between specific interventions and an outcome, the performed meta-analysis should be intended as a descriptive synthesis of the available published associations. This approach helps identify a state-of-the-art useful for planning new research endeavors. Thus, the model showing the pooled effect size derived from the included meta-analyses (meta-analysis of meta-analyses) was considered the most suitable for the reporting purpose due to its easier readability. Accordingly, the presented meta-analysis has to be used to support the narrative synthesis of the included systematic reviews rather than to define precise associations between physical exercise and CRF.
We conducted a subgroup analysis based on tumor localization to explore differences in the effect size among different cancer populations and minimize statistical heterogeneity across studies. A new I2 value was estimated for each subgroup. The funnel plot was created to assess small-study effects for the outcome, considering that a funnel plot that is asymmetrical for the line of the summary effect suggests differences between the estimates derived from small and large studies. Specifically, a contour-enhanced funnel plot (1, 5, and 10% significance) was used to explore whether the asymmetry is due to publication bias or other factors. Data analyses were performed using STATA 16 software [Citation35].
Results
Search results and characteristics of the included systematic reviews
The selection process and reasons for exclusion are presented in . Overall, 16 systematic reviews were assessed for eligibility in this study. Following the quality assessment process, 11 articles were finally included; however, no meta-analyses were available for one study; then, ten articles were included in the pooled meta-analysis.
Figure 1. Selection process (flow diagram). From Page et al. [Citation36]. For more information, visit http://www.prisma-statement.org/.
![Figure 1. Selection process (flow diagram). From Page et al. [Citation36]. For more information, visit http://www.prisma-statement.org/.](/cms/asset/c6857f14-2779-4abf-951e-dcdd33626c6e/ionc_a_1962543_f0001_c.jpg)
The ten systematic reviews, including meta-analyses, encompass results from 187 primary studies, but only 149 studies were focused on testing physical exercise for improving cancer-related fatigue, encompassing a total of 16,143 participants. All the primary studies included in the 11 systematic reviews were randomized controlled trials according to the research question’s nature. Two Cochrane reviews were suitable for the inclusion criteria [Citation37,Citation38]. Characteristics of the included systematic reviews are presented in .
Table 1. Characteristics of the included studies.
Characteristics of participants
Studies enclosed in the selected systematic reviews involved male and female adult participants affected by different solid tumors during and/or after cancer treatment. Systematic reviews, including studies targeted to patients with hematological malignancy, were excluded during the selection process. Participants included in the studies received exercise training during and/or after cancer treatment, including chemotherapy, radiotherapy, hormonotherapy, surgery, or a combination of them.
Characteristics of interventions
The systematic reviews include studies focused on the effect of physical training for improving cancer-related fatigue in comparison with usual care or no treatment [Citation37,Citation39–45] or a combination with low to high-intensity exercise [Citation46] and stretching or relaxation exercise [Citation38]. Physical training consisted of a combination of aerobic and resistance exercise [Citation37–43,Citation45,Citation46], aerobic and anaerobic exercise [Citation42,Citation43,Citation47], or exclusively aerobic exercise [Citation44]. The exercise training was supervised and/or home-based [Citation37,Citation38, Citation41,Citation45–47]. The frequency and duration of training programs differed across trials included in the systematic reviews, from 10 to 120 min, 1–6 times per week, for an overall period of 2–52 weeks, combining moderate to high intensity.
Outcome and measurements
The assessed outcome was cancer-related fatigue, measured with eleven types of multidimensional fatigue scales and subscales at different time points (short, long term follow up and at final assessment): FACT-F (Functional Assessment of Cancer Therapy: Fatigue), FACT-An (Functional Assessment of Cancer Therapy-Anemia), FACIT-F (Functional Assessment of Chronic Illness Therapy-Fatigue), PFS (Piper Fatigue Scale), R-PFS (Revised Piper Fatigue Scale), BFI (Brief Fatigue Inventory), SAS (Symptom Assessment Scale), POMS-F (Profile of Moods States Fatigue subscale), SCFS (Schwartz Cancer Fatigue Scale), FSS (Fatigue Severity Scale), MFI (Multidimensional Fatigue Inventory), FS (Fatigue Scale), LASA (Linear Analog Scale Assessment), TOI-F (Trial outcome index-fatigue), EORTC QLQ C30/FA13 (European Organization for Research and Treatment of Cancer Quality-of-life/fatigue subscale), and FAQ (Fatigue Assessment Questionnaire).
Risk of bias of the included systematic reviews
The authors estimated the risk of bias of the sixteen systematic reviews independently using the ROBIS tool [Citation30]. We judged eleven systematic reviews at low risk of bias [Citation37,Citation39–47], three systematic reviews at high risk of bias [Citation48–50] because the review process was not systematic, and two systematic reviews as unclear [Citation48,Citation51] because of a considerable number of missing data. Overall, the lower quality was found in the study eligibility criteria, identification, and selection phases. In particular, eligibility criteria were not fully described, neither the search strategies and reasons for restrictions. The results of the methodological quality assessment are reported in and Supplementary File 2.
The effect of physical training on cancer-related fatigue
A total of 16 meta-analyses were retrieved from 10 systematic reviews and pooled to estimate the magnitude of the effect of physical training on cancer-related fatigue. We did not compute one study’s results in the metanalysis as it was focused on patients with colorectal cancer who underwent physical training after surgery [Citation46]. The pooled estimate indicated a statistically significant difference in fatigue between the exercise intervention and control groups (SMD = −0.33; 95% CI = −0.43, −0,23, I2 = 64%) (), indicating a slightly positive effect of physical training on fatigue reduction. The subgroup analysis () based on tumor localization proved a slightly greater effect of physical training on fatigue in breast cancer patients (SMD = −0.36; 95% CI = −0.57, −0.15), compared to patients with prostate cancer (SMD = −0.34; 95% CI = −0.45, −0.22) with an overall effect (SMD) equal to −0.35 (−0.49, −0.21). However, no significant statistical difference was observed among the two groups (p = 0.85). The studies including breast cancer patients presented more variability (Tau2 = 0.06, I2 = 78.32%, H2 = 4.61) than the prostate group (Tau2 = 0.000, I2 = 0%, H2 = 1).
The asymmetric-shaped funnel plot (Supplementary File 3) suggested a slight risk of publication bias, in particular for studies with a small sample size, rather than other reasons, such as variability between the studies. The slight/moderate publication bias was confirmed by the contour-enhanced funnel plot, which showed missing studies in non-significant regions. Accordingly, funnel plots within groups remain asymmetric, corroborating the evidence of a publication bias.
Discussion
The role of physical training in cancer symptom management has been widely recognized as a part of routine cancer care. This systematic review of systematic reviews provides an overview of all current updated evidence on the effect of physical training on cancer-related fatigue in adults. Considering the substantial number of systematic reviews on this topic, supplying a clear perspective on the beneficial effects of physical exercise on fatigue reduction in different cancer populations is imperative for addressing the appropriate management strategy and direct future research.
The aggregate effect of physical training on fatigue suggests that exercise programs (aerobic anaerobic and resistance training) implemented during or after cancer treatments, such as chemotherapy, radiotherapy, or hormonotherapy have a small positive impact on fatigue reduction to usual care. Despite these benefits, only a minority of cancer patients are adequately active [Citation52,Citation53] and adhere to physical activity guidelines [Citation54]. Several barriers have been identified as poor physical exercise adherence predictors, including physical factors, fear, and low willingness to change physical exercise behavior [Citation55]. Furthermore, healthcare providers reported a modest attitude and knowledge to promote physical activity programs [Citation56]. A substantial stakeholders’ action to create sustainable multidisciplinary cancer rehabilitation models as a treatment option, equally to cardiac rehabilitation pathways and the infrastructure for a cultural adaptation, is required [Citation57,Citation58].
The frequency and duration of training programs highly differed across trials included in the systematic reviews, from 10 to 120 min, 1–6 times per week, for an overall period of 2–52 weeks, combining moderate to high intensity. For this reason, it was unfeasible to target a specific effective program. However, given the high heterogeneity of cancer populations and presentations, programs should be individually tailored, and a standard program proposal is not suggested [Citation59]. Physical activity recommendations should be integrated into patients’ experiences within the context of a patients’ life, recognizing the impairing effects of cancer treatments, home and working life, and patient’s physical and psychological needs [Citation7]. This approach might result in optimizing participation in exercise interventions [Citation60]. Accordingly, cancer patients’ physical training preferences vary considerably, especially if we consider different cancer populations [Citation52,Citation61–63].
Our results demonstrated a small effect of physical training in reducing fatigue in breast and prostate cancer patients. However, the small difference in the physical exercise effect between the two-cancer diagnosis and the overall estimation of all cancer diagnoses, peculiarities, and specific cancer populations’ barriers need to be considered in addressing the interventions [Citation64]. Specifically, these results might suggest additional potential benefits in delivering tailored cancer-specific intervention programs. However, additional valuable research needs to be conducted to enable an overall synthesis of the effect of physical exercise on other cancer diagnoses.
Finally, the small aggregate effects of physical exercise on reducing cancer-related fatigue and the small effects of breast or prostate cancer patients might reflect some weaknesses in how the outcome was measured. More precisely, cancer-related fatigue has been measured in the primary studies of the included systematic reviews by using 16 different tools. Previous research highlighted that cancer-related fatigue is a multidimensional symptom that involves physical and mental aspects [Citation17]. However, the several available tools for assessing cancer-related fatigue show a different number of domains, varying from 2 to 5 [Citation17]. Theoretically, the several domains of cancer-related fatigue might be susceptible to improvements by being modulated from different interventions’ effects. For instance, it is reasonable that physical exercise might mainly enhance the physical dimension of cancer-related fatigue, while other dimensions, such as mental fatigue, might be more susceptible to psychosocial interventions. Thus far, given the multitude of available tools, most of the available meta-analyses synthesized the effects of physical exercise on the overall score of cancer-related fatigue. For this reason, future research should clarify the quality of the evidence regarding the validity and reliability of the several tools to measure cancer-related fatigue for providing a theory-grounded base for clinicians and researchers who require determining which domain of fatigue is more susceptible to physical exercise. The future critical appraisal of the characteristics of the available tools for measuring cancer-related fatigue could help to clarify with more precision in which domains cancer-related fatigue could be defined, as currently, there is no consensus about this aspect [Citation17].
Limitations
This study has several limitations. Firstly, since we aimed to include high-quality evidence, only two specific cancer clusters (breast and prostate patients) resulted from the selection process and were suitable for the subgroup analysis. Secondly, comparisons across the systematic reviews revealed no univocal operational definition and categorization of unidimensional and multidimensional fatigue measures, and multiple scales have been utilized in the clinical trials. Thirdly, the usual care or conventional treatment approaches vary across the studies related to the local standard of care. This heterogeneity generated concerns about the synthesis and interpretability of the systematic review’s results. Nevertheless, this is the first systematic review of reviews and metanalysis, which provides a clear understanding of physical exercise’s impact on cancer-related fatigue, focusing on specific cancer populations. Forth, the design adopted for this systematic review of systematic reviews is not suitable for detecting associations between CRF and physical exercise over time by considering crucial time points, such as during treatment and after treatment. Having this information is pivotal to precisely ascertain when physical exercise might be more effective in reducing CRF. For this reason, future reviews designed to capture longitudinal data are required.
Conclusions
This study presents a quantitative synthesis of the available systematic reviews and meta-analyses on the effect of physical exercise on cancer-related fatigue to frame the current knowledge on this topic comprehensively. Although the effect sizes are small, there is consistent empirical evidence to endorse the implementation of physical exercise during and after treatments as part of cancer care, particularly for breast cancer survivors; for this reason, this evidence should be considered in planning care paths. Further research should focus on frameworks’ implementation to deliver tailored interventions.
Author contributions
SB and RC were responsible for the conception and design of the systematic review. SB, RC conducted the database search and data collection. RC and SB were responsible for the data analysis. SB, RC, CA were involved in the interpretation of results and drafting the article. All the authors revised the manuscript critically and approved the final version.
Supplemental Material
Download JPEG Image (444.6 KB)Supplemental Material
Download MS Word (15.4 KB)Supplemental Material
Download MS Word (15.3 KB)Disclosure statement
No potential competing interest was reported by the author(s).
Additional information
Funding
References
- Ma Y, He B, Jiang M, et al. Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud. 2020;111:103707.
- McCabe RM, Grutsch JF, Braun DP, et al. Fatigue as a driver of overall quality of life in cancer patients. PLOS One. 2015;10(6):e0130023.
- Groenvold M, Petersen MA, Idler E, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105(2):209–219.
- Wyatt G, Sikorskii A, Tesnjak I, et al. Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Support Care Cancer. 2015;23(11):3183–3191.
- Won HS, Sun DS, Choi JY, et al. Factors associated with treatment interruption in elderly patients with cancer. Korean J Intern Med. 2019;34(1):156–164.
- Mina DS, Langelier D, Adams SC, et al. Exercise as part of routine cancer care. Lancet Oncol. 2018;19(9):e433–e436.
- The Lancet Oncology. Exercise and cancer treatment: balancing patient needs. Lancet Oncol. 2018;19:715.
- Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009;10(4):381–391.
- Salakari MRJ, Surakka T, Nurminen R, et al. Effects of rehabilitation among patients with advances cancer: a systematic review. Acta Oncol. 2015;54(5):618–628.
- Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104.
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–654.
- Friedenreich CM, Wang Q, Neilson HK, et al. Physical activity and survival after prostate cancer. Eur Urol. 2016;70(4):576–585.
- Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534.
- NCCN. Guidelines version 2.2018 cancer-related fatigue. Plymouth Meeting (PA); 2018.
- Mroz EA, Rocco JW. The challenges of tumor genetic diversity. Cancer. 2017;123(6):917–927.
- National Cancer Institute. 2015 Strategic priorities symptom management & Quality of Life Steering Committee (SxQoL SC). Bethesda (MD); 2015.
- de Raaf PJ, de Klerk C, van der Rijt CCD. Elucidating the behavior of physical fatigue and mental fatigue in cancer patients: a review of the literature. Psychooncology. 2013;22(9):1919–1929.
- Smith V, Devane D, Begley CM, et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15.
- Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1:7.
- Chien PF, Khan KS, Siassakos D. Registration of systematic reviews: PROSPERO. BJOG. 2012;119(8):903–905.
- Jenkins M. Evaluation of methodological search filters-a review. Health Info Libr J. 2004;21(3):148–163.
- National Library of Medicine. PubMed® special queries 2019.
- Bramer WM, de Jonge GB, Rethlefsen ML, et al. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531–541.
- Richards D. Handsearching still a valuable element of the systematic review. Evid Based Dent. 2008;9(3):85.
- Leblanc TW, Abernethy AP, Casarett DJ. What is different about patients with hematologic malignancies? A retrospective cohort study of cancer patients referred to a hospice research network. J Pain Symptom Manage. 2015;49(3):505–512.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Whiting P, Savović J, Higgins JPT, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0. Chichester (UK): John Wiley & Sons; 2019.
- Banzi R, Cinquini M, Gonzalez-Lorenzo M, et al. Quality assessment versus risk of bias in systematic reviews: AMSTAR and ROBIS had similar reliability but differed in their construct and applicability. J Clin Epidemiol. 2018;99:24–32.
- Whiting Kleijnen P, Higgins J, Reeves B, et al. ROBIS: tool to assess risk of bias in systematic reviews. Guidance on how to use ROBIS. Bristol (UK): University of Bristol; 2016.
- Campbell M, Egan M, Lorenc T, et al. Considering methodological options for reviews of theory: illustrated by a review of theories linking income and health. Syst Rev. 2014;3:114.
- DerSimonian R, Kacker R. Random-effects model for Meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114.
- Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
- StataCorp. Stata statistical software: release 16. College Station (TX): StataCorp LLC; 2019.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145
- Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001.
- Juvet LK, Thune I, Elvsaas IKO, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177.
- Bo Y, W J. Effects of exercise on cancer-related fatigue and quality of life in prostate cancer patients undergoing androgen deprivation therapy: a meta-analysis of randomized clinical trials. Chinese Med Sci J. 2017;32:13–21.
- Dennett AM, Peiris CL, Shields N, et al. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother. 2016;62(2):68–82.
- Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and Meta-analysis. BMC Cancer. 2015;15:77.
- Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother. 2015;61(1):3–9.
- Zou LY, Yang L, He XL, et al. Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumor Biol. 2014;35(6):5659–5667.
- Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, et al. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol. 2010;22(3):208–221.
- Cramer H, Lauche R, Klose P, et al. A systematic review and Meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care. 2014;23(1):3–14.
- Tian L, Lu HJ, Lin L, et al. Effects of aerobic exercise on cancer-related fatigue: a meta-analysis of randomized controlled trials. Support Care Cancer. 2016;24(2):969–983.
- Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:479–494.
- Lee J, Lee MG. Effects of exercise interventions on breast cancer patients during adjuvant therapy: a systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. 2020;43(2):115–125.
- Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32(4):335–346.
- Nakano J, Hashizume K, Fukushima T, et al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther. 2018;17(4):1048–1058.
- Avancini A, Pala V, Trestini I, et al. Exercise levels and preferences in cancer patients: a cross-sectional study. IJERPH. 2020;17(15):5351–5322.
- Sweegers MG, Boyle T, Vallance JK, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act. 2019;16(1):66.
- Coletta AM, Marquez G, Thomas P, et al. Clinical factors associated with adherence to aerobic and resistance physical activity guidelines among cancer prevention patients and survivors. PLOS One. 2019;14(8):e0220814.
- Ormel HL, van der Schoot GGF, Sluiter WJ, et al. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27(3):713–724.
- Hardcastle SJ, Kane R, Chivers P, et al. Knowledge, attitudes, and practice of oncologists and oncology health care providers in promoting physical activity to cancer survivors: an international survey. Support Care Cancer. 2018;26(11):3711–3719.
- Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–484.
- Berger AM, Mitchell SA, Jacobsen PB, et al. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA. CA Cancer J Clin. 2015;65(3):190–211.
- Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723.
- IJsbrandy C, Hermens RPMG, Boerboom LWM, et al. Implementing physical activity programs for patients with cancer in current practice: patients' experienced barriers and facilitators. J Cancer Surviv. 2019;13(5):703–712.
- McGowan EL, Speed-Andrews AE, Blanchard CM, et al. Physical activity preferences among a population-based sample of colorectal cancer survivors. Oncol Nurs Forum. 2013;40(1):44–52.
- Philip EJ, Coups EJ, Feinstein MB, et al. Physical activity preferences of early-stage lung cancer survivors. Support Care Cancer. 2014;22(2):495–502.
- Leach HJ, Devonish JA, Bebb DG, et al. Exercise preferences, levels and quality of life in lung cancer survivors. Support Care Cancer. 2015;23(11):3239–3247.
- Wurz A, St-Aubin A, Brunet J. Breast cancer survivors' barriers and motives for participating in a group-based physical activity program offered in the community. Support Care Cancer. 2015;23(8):2407–2416.