Abstract
Introduction
50% of patients with locally advanced HNSCC eventually present with disease recurrence or metastasis. Interaction of programmed cell death protein 1 (PD-1) and its ligand, programmed death-ligand 1 (PD-L1), allows tumour cells to evade immune attack by inhibiting T-cell activation. PD-1/PD-L1 checkpoint inhibitors block this immunosuppressive effect. This study aims to investigate the efficacy of anti-PD-1/PD-L1 agents for recurrent/metastatic (R/M) HNSCC in terms of survival, toxicity, and response. It will test the hypothesis that immunotherapy improves treatment outcomes for R/M HNSCC patients.
Material and methods
Studies were identified through an electronic search of databases EMBASE and Medline. Data on survival, response and toxicity following PD-1/PD-L1 inhibition was extracted from included studies and compared. A subgroup meta-analysis compared these outcomes in PD-1/PD-L1 inhibition versus the standard of care (SOC).
Results
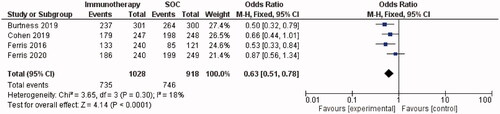
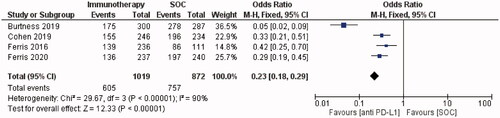
Thirteen studies (n = 1798) were included in this review. Overall survival following PD-1/PD-L1 checkpoint inhibition ranged from 6 to 13 months. The most common treatment-related adverse events (TRAEs) were fatigue, hypothyroidism and nausea; Grade ≥3 TRAEs occurred in 13% of patients. Meta-analysis of RCTs showed that anti-PD-1/PD-L1 agents improved survival and reduced toxicity compared to the SOC. This was demonstrated by a 37% lower risk of death (OR = 0.63, 95% CI = 0.51–0.78, I2 = 18%, p ≤ 0.0001) and a 77% lower risk of any-grade TRAEs (OR = 0.23, 95% CI = 0.18–0.29, I2 = 90%, p ≤ 0.00001) with immunotherapy versus SOC.
Discussion
Based on the observed safety and efficacy, PD-1/PD-L1 checkpoint inhibition improves treatment outcomes for R/M HNSCC patients. PD-1/PD-L1 inhibitors significantly prolonged survival and reduced toxicity compared to the SOC, however further randomised trials are needed to investigate their role in HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide [Citation1] and the incidence is rising [Citation2]. Approximately 60% of patients present with locally advanced (LA) disease at diagnosis [Citation3], with a 50% recurrence rate [Citation4]. Recurrent or metastatic (R/M) HNSCC is associated with a poor prognosis, and few effective treatment options are available. Most patients with localised primary recurrence receive palliative systemic therapy, as few patients with locoregional recurrence can be salvaged by surgery or reirradiation [Citation3].
The standard of care (SOC) systemic therapy for R/M HNSCC is platinum-based chemotherapy with fluorouracil (5-FU) and cetuximab [Citation5]. Median survival for patients treated with chemotherapy alone is less than a year. The EXTREME regimen is commonly used in the first-line treatment of R/M HNSCC, combining 5-FU with cisplatin/carboplatin and cetuximab followed by maintenance cetuximab. The EXTREME regimen has a median overall survival (OS) of 10 months for R/M HNSCC patients [Citation1]. For patients that are ineligible for the EXTREME regimen, taxanes and methotrexate are some of the few therapeutic options available. The median OS drops to 6 months with these drugs [Citation1]. This poor outcome evokes the need for novel treatment options in the management of R/M HNSCC.
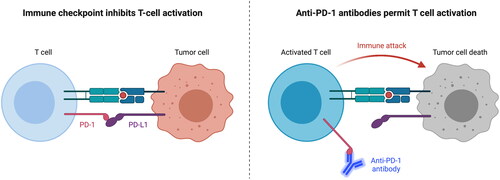
Immunotherapy has shown positive results in recent research [Citation6,Citation7]. The rationale behind immune checkpoint inhibition in HNSCC comes from the association of HNSCC with deficiencies of the immune system such as altered natural killer cell function and impairment of tumour-infiltrating T lymphocytes exhibiting an important impact on clinical outcomes [Citation1]. The immune checkpoint pathway constitutes an important mechanism of tumour immune escape, and is regulated by interactions between ligands and receptors, such as between programmed cell death protein 1 (PD-1) and its ligands programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2). In the healthy immune system, the PD-1 receptor functions as an immune checkpoint and is expressed primarily on the surface of activated T cells [Citation8]. The binding of PD-1 with PD-L1 results in inhibition of T cell activation and limits the response to inflammation, thus acting as an immune ‘off’ switch (). Approximately 60% of HNSCC tumour cells express high levels of PD-L1, creating an immunosuppressive micro-environment around the tumour, protecting it from immune attack [Citation9–11]. Thus, immune checkpoint inhibitors aim to activate and allow the immune system to attack the tumour cells. Despite the high percentage of tumour cells expressing PD-L1, there are challenges associated with using PD-L1 as a predictive biomarker of response to immune checkpoint inhibitors. Intratumour heterogeneity of PD-L1 expression between tumour biopsies can lead to ambiguous results on PD-L1 status [Citation12].
Anti-PD-1 agents Nivolumab and Pembrolizumab were the first two immune checkpoint inhibitors to be approved by the US Food and Drug Administration in 2016 for the treatment of R/M HNSCC patients with disease progression on or after platinum-based therapy [Citation4]. They work by binding to the PD-1 receptor to inhibit the reaction between PD-1 and its ligands PD-L1/PD-L2. In contrast to anti-PD-1 agents, anti-PD-L1 agents bind PD-L1 on tumour cells and block interaction with PD-1 and B7-1. These agents do not bind to PD-L2, that is, they do not prevent interaction between PD-1 and PD-L2, therefore potentially reducing PD-L2-mediated autoimmune toxicity [Citation11]. Anti-PD-L1 agents Avelumab, Atezolizumab, and Durvalumab have received approval for treatment of a variety of solid tumours [Citation13]. Durvalumab has been investigated in R/M HNSCC, demonstrating encouraging anti-tumour activity and survival for this patient cohort [Citation6,Citation7].
Studies have shown that by blocking the interaction between the PD-1 receptor and its ligands, anti-PD-1 agents may reactivate tumour immune surveillance and induce anti-tumour activity. A phase I clinical trial suggested that Pembrolizumab is safe and tolerable for R/M HNSCC, supporting further research into Pembrolizumab as a novel treatment for advanced head and neck cancer (HNC). Data obtained from this study support the hypothesis that PD-L1 is a biomarker for the prediction of tumour response to these immune checkpoint inhibitors [Citation14]. Compared to the SOC, anti-PD-1 agents have demonstrated superiority in phase III randomised control trials (RCTs) for this patient cohort [Citation5]. Nivolumab has demonstrated anti-tumour activity in multiple tumour types, prior to investigation of its role in HNC [Citation15,Citation16].
With the limited treatment options and poor prognosis for R/M HNSCC patients, continued research is needed to improve patient outcomes. A recent publication concluded the need for a higher level of evidence-based randomised trials to investigate the efficacy of checkpoint inhibition for R/M HNSCC [Citation17]. This research aims to add to the current gap in the evidence by combining current knowledge and evaluating the clinical potential of PD-1/PD-L1 checkpoint inhibition for R/M HNSCC.
The primary aim of this research is to critically review the literature and extract and analyse data on OS, progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (TRAEs), to determine the efficacy and safety of PD-1/PD-L1 checkpoint inhibitors. The secondary aim is to compare the efficacy of PD-1/PD-L1 inhibitors to the SOC for the treatment of R/M HNSCC by carrying out a subgroup meta-analysis of RCTs. Data on survival, toxicity, and response will be extracted and compared between these treatment modalities using forest plots.
Material and methods
An electronic search of the databases Embase and Medline was carried out to identify relevant studies. The complete search strategy that was input into the databases is available in the Supplementary Appendix A. A final revision of the search was carried out on 5th June 2020.
Prospective and retrospective studies investigating PD-1/PD-L1 checkpoint inhibitors for R/M HNSCC in human clinical trials were eligible for inclusion in this study. Both randomised and non-randomised trials were included. In single-arm studies, participants were treated with an anti-PD-1/PD-L1 agent only. In double-arm studies, the control arm was treated with the investigator’s choice of chemotherapy (standard of care therapy). Studies investigating checkpoint inhibitors given in combination with another treatment were excluded, except for when data from a monotherapy arm was available. Phase III RCTs generated in the search that assesses the use of anti-PD-1/PD-L1 monotherapy versus the SOC were separated for inclusion in a subgroup meta-analysis.
Eligible studies included participants that were ≥18 years old with a confirmed diagnosis of R/M HNSCC, had measurable disease based on Response Evaluation Criteria in Solid tumours (RECIST, version 1.1), Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and adequate organ function [Citation18]. There were no eligibility criteria based on biomarker expression, participants could be human papilloma virus (HPV) positive/negative, or PD-L1 positive/negative. Patients were excluded if they had additional progressing malignancies, active or previous brain metastases, autoimmune disease requiring systemic immunosuppression, spinal cord compression, leptomeningeal carcinomatosis, known hepatitis B or C virus infection, or history of the human immunodeficiency virus (HIV).
Outcome measures
OS, PFS, ORR, and incidence of adverse events (any grade and grade ≥3) were the primary outcomes of this review. OS was defined across studies as the time from allocation to death due to any cause. Progression-free survival was defined as the time from allocation to progressive disease according to RESIST version 1.1 and modified RECIST or death due to any cause, whichever occurred first. The response was defined as the proportion of patients with complete or partial response, assessed using RESIST v1.1. Adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and version 4.03. All included studies reported on at least one of these outcome measures.
Statistical analysis
All studies used Kaplan–Meier statistics for estimates of PFS, OS, and response duration, except for one study by Segal et al. [Citation7]. In most studies, confidence intervals (CI) and p-values were estimated with the exact binomial method. OS and PFS were compared by stratified log-rank tests in four studies [Citation19–23]. Ferris et al. used the Cochrane-Mantel-Haenszel method to calculate the odds ratio (OR) and associated CIs for tumour response in two studies [Citation20,Citation24]. Stratified log-rank tests were used to compare OS and PFS between treatment groups across RCTs [Citation19,Citation20,Citation23,Citation25]. In three studies, CIs for median survival times were calculated using the Brookmeyer and Crowley method [Citation19,Citation20,Citation24]. Ferris et al. [Citation20] used the Borgan and Liestøl method to calculate CIs for survival at specific time points. Cox proportional-hazards models were used to estimate hazard ratios and calculate the associated CIs in included RCTs [Citation19,Citation20,Citation23–25].
All studies were included in the qualitative review and a subgroup of 4 RCTs was further analysed in a meta-analysis. The remaining studies were excluded from meta-analysis as the data was not randomised. Meta-analysis of eligible RCTs was conducted using RevMan version 5.3 software (available from the Cochrane community). Outcomes were considered as statistically significant if p < 0.05.
Results
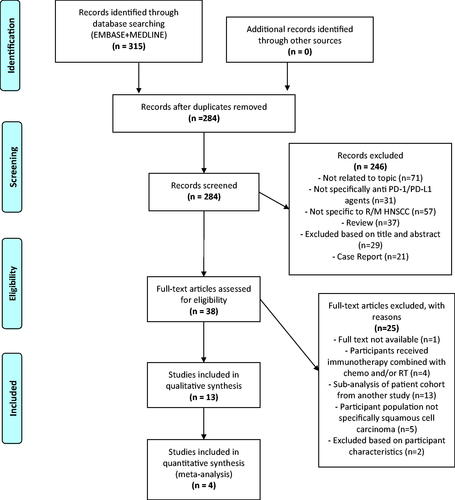
13 studies, including 4 RCTs were eligible for inclusion in the qualitative literature review. 4 RCTs were eligible for subgroup meta-analysis based on the pre-defined inclusion criteria. A PRISMA flowchart in displays the study selection process and reasons for the exclusion of studies. Where multiple publications reported on the same study, the publication with the most complete data was selected. Thirteen studies involving 1798 PD-1/PD-L1 inhibitor-treated patients were included in this review. PD-1 inhibitor Pembrolizumab was investigated in five studies (n = 911) and Nivolumab in four studies (n = 406). Four studies investigated PD-L1 inhibitor Durvalumab (n = 481). The characteristics of included studies are summarised in Supplementary Appendix B.
Figure 2. Prisma flowchart of systematic literature search. Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.p. For more information, visit www.prisma-statement.org.
Risk of bias in included studies
The quality of the four RCTs was assessed using the Jadad et al. scale. Out of 5 questions (5 points), all studies scored 3, as blinding could not be undertaken (Supplementary Appendix C). Quality analysis of included studies using the Downs and Black checklist yielded scores of 15–23; meaning studies are of limited to good quality (Supplementary Appendix D).
Effects of interventions
Overall survival
All studies investigated the survival of PD-1/PD-L1 inhibitor-treated patients. Median reported OS ranged from 6 months [Citation22] to 13 months [Citation14]. The subgroup meta-analysis demonstrated a statistically significant improvement in survival with immunotherapy versus SOC. Forest plots demonstrated a 37% lower risk of death in PD-1 inhibitor-treated patients than in patients treated with the SOC (OR = 0.63, 95% CI = 0.51–0.78, I2 = 18%, p ≤ 0.0001; ).
Progression-free survival
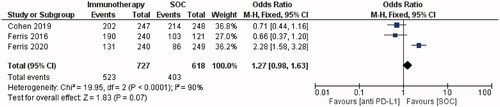
Median PFS was reported in all studies, ranging from 1.4 months [Citation7] to 5.9 months [Citation26]. A median PFS of 1.9–2.1 months was reported in eight studies [Citation8,Citation14,Citation20,Citation22,Citation24,Citation25,Citation27,Citation28]. The subgroup meta-analysis analysed progression-free survival in three out of four RCTs [Citation19,Citation20,Citation25], based on the availability of data. Forest plots showed that PD-1 inhibitor-treated patients had a 27% higher chance of disease progression than patients receiving the SOC, however, this difference was not statistically significant (OR = 1.27, 95% CI = 0.98–1.63, I2 = 90%, p = 0.07; ).
Response rate
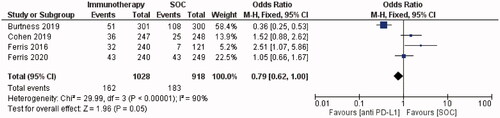
ORR data were available from all studies and ranged from 6.5% [Citation7] to 21.8% [Citation26]. In the subgroup meta-analysis, an objective response occurred more frequently in patients who received SOC versus immunotherapy. Patients receiving anti-PD-1 immunotherapy were 21% less likely to have an objective response than those receiving the SOC. (OR = 0.79, 95% CI = 0.62–1.00, I2 = 90%, p = 0.05; ).
Adverse events
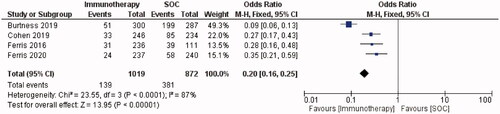
TRAEs were reported in 1011/1701 (59%) of PD-1/PD-L1-treated patients across twelve studies (). One study did not report the incidence of adverse events [Citation21]. The most common any-grade TRAEs were fatigue [Citation7,Citation8,Citation14,Citation20,Citation22–24,Citation27], hypothyroidism [Citation19,Citation25,Citation26] and nausea [Citation28]. 13% (221/1,701) of PD-1/PD-L1 inhibitor-treated patients experienced grade ≥3 TRAEs across 12 studies. Cohen et al. [Citation19] reported that 63% and 84% of patients experienced any-grade TRAEs in the Pembrolizumab and SOC groups, respectively. The percentage of patients with grade ≥3 TRAEs was also higher in the SOC group (36%) than in the Pembrolizumab group (13%). Ferris et al. [Citation20] reported similar results, with the incidence of grade ≥3 TRAEs decreasing from 35.1% to 13.1% with Nivolumab versus SOC. Forest plots showed that the PD-1 inhibitor group had a 77% lower risk of any-grade TRAEs than the SOC group (OR = 0.23, 95% CI = 0.18–0.29, I2 = 90%, p ≤ 0.00001; ). Forest plots demonstrated that the PD-1 inhibitor group had an 80% lower risk of grade ≥3 TRAEs than the SOC group (OR = 0.20, 95% CI = 0.16–0.25, I2 = 87%, p < 0.00001; ).
Table 1. Results from included studies on the effects of anti PD-1/PD-L1 immunotherapy on R/M HNSCC.
Treatment-related deaths were reported in six studies. Deaths were reported due to pneumonitis n = 3 [Citation20,Citation23,Citation24,Citation27], large intestine perforation n = 1, malignant neoplasm progression n = 1 and Stevens-Johnson syndrome n = 1 [Citation19], hypercalcaemia n = 1 [Citation20,Citation24], disseminated vascular coagulation n = 1 [Citation23], autoinflammatory disease n = 1 [Citation23], and unspecified n = 5 [Citation19,Citation25].
Discussion
This research examines the effect of PD-1/PD-L1 immune checkpoint inhibition on R/M HNSCC. A qualitative review of thirteen studies found that treatment of R/M HNSCC patients with PD-1/PD-L1 inhibitors resulted in a median OS of between 6 and 13 months. Siu et al. [Citation22] reported the lowest OS (6 months), patients received 10 mg/kg anti-PD-L1 agent Durvalumab every 2 weeks. Seiwert et al. [Citation14] reported the highest median OS (13 months), anti-PD-1 agent Pembrolizumab was administered at 10 mg/kg every 2 weeks. Response rates of participants also varied amongst included studies. ORR of patients who received anti-PD-L1 agent Durvalumab ranged from 6.5% to 17.9%. Response to anti-PD-1 agents Pembrolizumab and Nivolumab ranged from 14.6% to 18% and 13.3% to 21.8%, respectively. It should be noted that the highest objective response (21.8%) was reported in the retrospective study by Hori et al. [Citation26], in which the dose and timeframe of Nivolumab administration were not reported. The differences seen in the results between studies may have been due to variations in the treatment regimen, or drug and dose administered. Response to immunotherapy can vary if treatment is given as a first or second-line therapy, however, for patients included in this review this is their first exposure to immunomodulation. Performance status could also have a significant impact on response to treatment for patients. However, in this study, patients eligible for inclusion had a performance status of ECOG 0–1 except for less than 2% of the patients in the Nivolumab study. Therefore, patients with a performance status of 2 or greater were not represented in a meaningful amount for further analysis. Thus, there is a need for further phase 4 data to validate the influence of factors such as performance status and age on response to treatment.
The subgroup quantitative meta-analysis of 4 RTCs showed an improved objective response with SOC versus checkpoint inhibition, although the result was of borderline significance (p = 0.05). Three out of four RCTs represented in the meta-analysis showed a higher response rate with PD-1/PD-L1 inhibition versus the SOC [Citation19,Citation20,Citation25]. Burtness et al. demonstrated a higher objective response with cetuximab and chemotherapy versus Pembrolizumab (36% versus 17%) [Citation23]. However, in this study, Pembrolizumab alone was associated with more complete responses and an improved median response duration by more than 16 months versus the SOC [Citation23].
PD-L1 expression varied amongst participants in these studies. Siu et al. [Citation22] enrolled a PD-L1 low/negative participant population, and Seiwert et al. [Citation14] enrolled PD-L1 positive patients only. A subgroup meta-analysis showed an improvement in survival with checkpoint inhibition versus the SOC. Cohen et al. [Citation19] reported an increase in OS with Pembrolizumab (8.4 months) versus the SOC (6.9 months). In this study, 200 mg Pembrolizumab was administered every 3 weeks, compared with 10 mg/kg every 2 weeks in Seiwert et al.’s study in which the highest median OS was reported [Citation14]. Ferris et al. [Citation20] reported similar outcomes, with survival increasing from 5.1 months with SOC to 7.5 months with Nivolumab (3 mg/kg every 2 weeks). Nivolumab demonstrated prolonged survival benefit, as reported in a 2-year update from the same study [Citation24]. Anti-PD-L1 agent Durvalumab did not show a statistically significant survival benefit over the SOC in Ferris et al.’s RCT, with a median OS of 7.6 months with Durvalumab versus 8.3 months with the SOC [Citation25]. However, the median OS of Durvalumab reported in this study (7.6 months) is similar to the median OS of Nivolumab (7.5 months) and Pembrolizumab (8.4 months) in comparable patient populations [Citation19,Citation20]. Additionally, the 12-month survival rate was higher with Durvalumab (37%) versus the SOC (30.5%), and similar differences were seen with 18- and 24-month survival rates, suggesting a survival benefit favouring Durvalumab.
Six studies investigated OS stratified based on PD-L1 expression [Citation7,Citation8,Citation19,Citation20,Citation24,Citation25], demonstrating an increase in median OS for PD-L1 positive patients compared with PD-L1 negative patients. Ferris et al. [Citation25] reported a median OS of 9.8 months in PD-L1 positive patients treated with Durvalumab, compared with an OS of 7.6 months in PD-L1 negative patients. Chow et al. [Citation8] reported an OS of 9.9 months in PD-L1 positive patients following Pembrolizumab, almost double that reported in PD-L1 negative patients (4.9 months). Seiwert et al. [Citation14] and Zandberg et al. [Citation28] enrolled patients with PD-L1 positive tumour expression only. Zandberg et al. [Citation28] reported the highest median OS amongst all studies (13 months), which may be attributed to the inclusion of PD-L1 positive patients only. In these five studies, PD-L1 expression was associated with a higher ORR [Citation7,Citation8,Citation19,Citation20,Citation27]. This is consistent with findings that correlate PD-L1 expression with a higher response to anti-PD-1 immunotherapy in cancer sites such as advanced melanoma, non-small-cell lung cancer, colorectal cancer, and genitourinary cancer [Citation29,Citation30]. Burtness et al. [Citation23] reported a significantly higher OS with Pembrolizumab monotherapy versus the SOC amongst PD-L1 positive patients. PD-L1 expression is an important predictive biomarker of response to checkpoint inhibition. Standardisation of scoring and staining may allow for PD-L1 to become a definitive predictive biomarker for treatment response [Citation29]. However, a single biopsy may not be a reliable guide due to intra-tumour heterogeneity in PD-L1 expression [Citation12]. Scoring methods vary between laboratories and may need to be considered when evaluating PD-L1 expression, with both the tumour proportion score (TPS) and the combined positive score (CPS) commonly used. Variation has been observed in responders using the different scoring systems [Citation31]. Variation in scoring methods can also influence concordance between the different staining assays, with moderate to poor concordance shown for both TPS and CPS [Citation32].
HPV-positive HNC is associated with a more favourable prognosis than HPV-negative HNC [Citation33]. ORR of PD-1/PD-L1 inhibitor-treated patients was reported in six studies, stratified based on HPV status [Citation7,Citation8,Citation14,Citation20,Citation27,Citation28]. In most of these studies, HPV-positive patients had a numerically higher response rate than HPV-negative patients. Chow et al. [Citation8] and Zandberg et al. [Citation28] reported the highest increase in response in HPV-positive versus HPV-negative patients with an increase of 18.6% and 18%, respectively. Amongst the patient population with ≥25% PD-L1 expression in Zandberg et al.’s [Citation28] study, there was a higher reported OS (10.2 months vs 5.0 months) and PFS (3.6 months vs 1.8 months) in HPV-positive versus HPV-negative patients. Ferris et al. [Citation20] reported a higher OS with Nivolumab versus the SOC regardless of HPV (p16) or PD-L1 status; however, it was observed that patients with tumour PD-L1 ≥ 1% or p16-positive tumours (or both) may have a greater response to Nivolumab than those with PD-L1 < 1% or p16-negative tumours [Citation20]. However, this review presents conflicting evidence on the reliability of HPV as a predictive biomarker of response. Bauml et al. [Citation27] and Seiwert et al. [Citation14] reported similar response rates of Pembrolizumab-treated patients irrespective of HPV status. Segal et al. [Citation7] reported a numerically greater ORR in HPV-negative than HPV-positive patients. As previously mentioned, PD-L1 expression is a predictive biomarker of response to checkpoint inhibition. However, no association has been found between PD-L1 expression and HPV status. There is not enough evidence to confirm that HPV-status is a predictive biomarker of response to anti-PD-1/PD-L1 immunotherapy for R/M HNSCC. It has been suggested that identification of predictive biomarkers related to the efficacy of immune checkpoint inhibitors, such as PD-L1, in combination with HPV status, might provide further clinical benefit to patients with HNSCC [Citation34]. The rationale for further studies to validate the benefit of checkpoint inhibition for HPV-positive patients may be supported by the increased expression of membranous PD-L1 in HPV-positive HNSCC [Citation35].
PD-1/PD-L1 checkpoint inhibitors demonstrated a manageable safety profile. 59% of R/M HNSCC patients treated with PD-1/PD-L1 inhibitors experienced a TRAE of any grade. Bauml et al. [Citation27] reported the highest incidence of any-grade TRAEs (64%); the most common were fatigue, hypothyroidism, nausea, aspartate aminotransferase increase, and diarrhoea. Other common TRAEs across included studies were decreased appetite, rash, and anaemia. Across twelve studies, grade ≥3 TRAEs were reported in 13% of patients. Although this percentage is small, attention should be paid to the type and severity of such adverse events. Across six studies, grade ≥3 fatigue was reported as a common TRAE [Citation7,Citation19,Citation20,Citation22,Citation23,Citation25]. The most common grade ≥3 TRAE reported by Segal et al. [Citation7] and Zandberg et al. [Citation28] was an increase in gamma-glutamyl transferase (an enzyme that is found in high concentrations in the liver); occurring in 3.2% and 2.7% of patients, respectively. Administration of 10 mg/kg of Durvalumab was common across these two studies. Increased levels of gamma-glutamyl transferase have been reported as severe toxicity associated with Durvalumab treatment in other cancer sites, such as non-small-cell lung cancer [Citation36].
The improved safety profile of anti-PD-1 agents is demonstrated in the subgroup meta-analysis ( and ). There was a higher incidence of any-grade and grade ≥3 TRAEs with the SOC versus PD-1 checkpoint inhibitors in all included RCTs [Citation19,Citation20,Citation23,Citation25]. However, grade 3–5 adverse events occurred in 10.1–17% of PD-1-treated patients across these studies [Citation19,Citation20,Citation23,Citation25]. It is important to communicate the risk of TRAEs to patients before they begin treatment with PD-1/PD-L1 checkpoint inhibitors. Early recognition and management of TRAEs are vital to prevent severe complications.
This research investigated PD-1/PD-L1 checkpoint inhibition as monotherapy for R/M HNSCC. Studies combining these agents with another treatment such as chemotherapy or radiation therapy (RT) were excluded (), due to the paucity of published evidence to date in the recurrent/metastatic setting. However, ongoing research is focussing on immunotherapy as part of a multimodality approach to HNSCC treatment.
In conclusion, PD-1/PD-L1 inhibitors present a manageable safety profile and appear to be effective for the treatment of R/M HNSCC. In addition, this research indicated improved survival with PD-1/PD-L1 checkpoint inhibition in PD-L1 positive versus PD-L1 negative patients. PD-1/PD-L1 checkpoint inhibition showed definitive superiority compared to the SOC in terms of survival and toxicity outcomes but the findings of this review are limited by the low number of RCTs and the variations in endpoint reporting which limited the data synthesis. There is a need for continued research into the potential benefits of PD-1/PD-L1 inhibitors as part of a multimodality approach to R/M HNSCC treatment.
Supplemental Material
Download MS Word (25.5 KB)Supplemental Material
Download MS Word (14.2 KB)Supplemental Material
Download MS Word (19.7 KB)Supplemental Material
Download MS Word (12.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saleh K, Eid R, Haddad FG, et al. New developments in the management of head and neck cancer – impact of pembrolizumab. Ther Clin Risk Manag. 2018;14:295–303.
- Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol. 2018;57(9):1143–1151.
- Specenier P, Vermorken JB. Optimal treatment for recurrent/metastatic head and neck cancer. Annals of Oncology. 2010;21(7):vii252–vii261.
- Argiris A, Harrington KJ, Tahara M, et al. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7(72):72.
- Nan X, Gold KA, Cohen E. Immunotherapeutic approaches to the management of head and neck cancer. Oncology. 2018;32(12):617–619.
- Gupta A, Spreafico A, Balmanoukian AS, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann Oncol. 2016;27(6):vi328.
- Segal NH, Ou SI, Balmanoukian A, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer. 2019;109:154–161.
- Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in Biomarker-Unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845.
- Forster MD, Devlin M-J. Immune checkpoint inhibition in head and neck cancer. Front Oncol. 2018;8:310.
- Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–3304.
- Pai SI, Zandberg DP, Strome SE. The role of antagonists of the PD-1:PD-L1/PD-L2 axis in head and neck cancer treatment. Oral Oncol. 2016;61:152–158.
- Rasmussen JH, Lelkaitis G, Håkansson K, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer. 2019;120(10):1003–1006.
- Yu Y, Lee NY. JAVELIN head and neck 100: a phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Future Oncol. 2019;15(7):687–694.
- Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Ghanizada M, Jakobsen KK, Grønhøj C, et al. The effects of checkpoint inhibition on head and neck squamous cell carcinoma: a systematic review. Oral Oncol. 2019;90:67–73.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–656.
- Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167.
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867.
- Okamoto I, Sato H, Kondo T, et al. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer - a retrospective multicentre study. Acta Oto-Laryngologica. 2019;139(10):918–925.
- Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-Low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195–203.
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet. 2019;394(10212):1915–1928.
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
- Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–950.
- Hori R, Shinohara S, Kojima T, et al. Real-World outcomes and prognostic factors in patients receiving nivolumab therapy for recurrent or metastatic head and neck carcinoma. Cancers. 2019;11(9):06.
- Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–1549.
- Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–152.
- Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–876.
- Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142.
- Cohen E, Harrington K, Soulières D, et al. Analysis of efficacy outcomes based on programmed death ligand 1 (PD-L1) scoring techniques in patients with head and neck squamous cell carcinoma (HNSCC) from KEYNOTE-040. Ann Oncol. 2019;30:v452–v453.
- de Ruiter EJ, Mulder FJ, Koomen BM, et al. Comparison of three PD-L1 immunohistochemical assays in head and neck squamous cell carcinoma (HNSCC). Mod Pathol. 2021;34(6):1125–1132.
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138.
- Kim HS, Lee JY, Lim SH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat. 2016;48(2):527–536.
- Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-Associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741.
- Alvarez-Argote J, Dasanu CA. Durvalumab in cancer medicine: a comprehensive review. Expert Opin Biol Ther. 2019;19(9):927–935.