Abstract
Background
Colorectal cancer (CRC) has negative long-term impacts on survivors’ health and work capacity. We aimed to investigate specialized healthcare use and sickness absence and disability pension among CRC survivors and matched references.
Material and methods
In this longitudinal register-based cohort study, 6679 patients with a first primary CRC in 2008–2011 (when aged 18–62) and 26,716 CRC-free matched references were followed from 2 years before up to 5 years after diagnosis date. Mean numbers of hospital days and outpatient visits were illustrated for survivors and references for the 7-year period. Crude and adjusted mean numbers of sickness absence/disability pension net days were calculated for post-diagnosis Years 3 and 5.
Results
Survivors’ healthcare use was higher compared to their references throughout the 7 years around CRC diagnosis and was mostly due to CRC, secondary neoplasms, and digestive disorders. In Year 5, survivors had 1.94 mean outpatient visits and 2.13 mean inpatient days (compared to 1.00 and 0.82 for references, respectively). Survivors’ adjusted mean sickness absence/disability pension days amounted to 85 d in Year 3 and 77 in Year 5 (compared to 57 and 54 d in the references). Higher mean number of future days was found among women, lower-educated, foreign-born, with previous comorbidities or previous mental disorders. Those with many sickness absence days in 2 years pre-diagnosis had the highest number of future sickness absence/disability pension days.
Conclusions
Post-diagnostic healthcare use remained high among CRC survivors, mostly due to cancer (CRC and other neoplasms) and digestive diagnoses. Their sickness absence and disability pension decreased gradually over the period but remained higher than among references. Pre-morbid characteristics may be used in early work-related clinical planning for the survivors.
Introduction
Colorectal cancer (CRC) is the third most common cancer type worldwide [Citation1]. Survival rates of CRC are improving [Citation2], while the CRC incidence in high-income countries has increased significantly among people below 50 years [Citation3]. To adequately support working-aged CRC patients, knowledge of their health and work is of growing importance also for oncologists [Citation4–7].
Side- and long-term effects of cancer disease and treatment can worsen CRC survivors’ health and work capacity long after diagnosis [Citation8]. Both mental and somatic disorders, e.g., depression, fatigue, peripheral neuropathy, and bowel and urogenital dysfunction are common among survivors [Citation9,Citation10]. Additionally, frequent pre-diagnostic comorbidities among survivors [Citation9,Citation11–13] can lead to elevated levels of healthcare use. According to two studies, CRC survivors post-diagnosis had higher rates of primary healthcare visits, medication prescriptions, and referrals [Citation14,Citation15]. However, studies elucidating future diagnoses-specific healthcare use are lacking, with one exception: a 5-year follow-up study showed a higher risk of hospitalization due to new somatic diagnoses in CRC survivors than in their CRC-free references [Citation16]. To illustrate working-aged CRC survivors’ specific needs of healthcare use, we need comprehensive longitudinal studies on their diagnoses-specific healthcare use.
Besides leading to higher healthcare use, long-term health problems may negatively impact survivors’ work capacity; however, this is not much studied. Some studies have shown a higher risk of unemployment [Citation17], work discontinuation [Citation18], sickness absence [Citation19,Citation20], and disability pension [Citation19–21] among CRC survivors compared to the general population. Given the importance of paid work for cancer survivors [Citation22], longitudinal studies are needed to show the extent of post-diagnostic sickness absence and disability pension, overall and among survivor subgroups.
Hence, we conducted a longitudinal cohort study of healthcare use and sickness absence and disability pension among CRC survivors and matched references. Specific aims: (1) quantify the extent for inpatient and specialized outpatient healthcare use in CRC survivors and their matched references before and after diagnosis; (2) calculate mean sickness absence/disability pension days and analyze associations of CRC survivor’s characteristics with future sickness absence and disability pension at 3- and 5-year post-diagnosis and compare to references.
Material and methods
We performed a longitudinal cohort study (a 5-year follow-up of all from inclusion date (T0), and also including data from the retrospective 2 years before T0), using microdata from six nationwide registers, linked through the unique personal identity number assigned to all residents in Sweden [Citation23].
Study population
Colorectal cancer survivors
CRC survivors and cancer characteristics were identified from the Swedish Cancer Register. The register started in 1958, is held by the National Board of Health and Welfare, and has almost 99% of completeness [Citation24]. We selected all 6679 people diagnosed with a first primary CRC (not diagnosed at autopsy) in Sweden in 2008–2011 when aged 18–62 years. The date of cancer diagnosis was used as T0. Cancer type was assessed using the International Classification of Diseases, the tenth revision (ICD-10) [Citation25], with codes C18 for colon and C19–20 for rectal cancer. The Cancer Register was also used to exclude those with a previous CRC diagnosis.
Matched references
The matched population references were to be alive and registered as living in Sweden the year before the diagnosis year of the index patient, and without any previous CRC diagnosis according to the Cancer Register. References were sampled from the Longitudinal Integrated Databases for Health Insurance and Labor Market Studies (LISA), held by Statistics Sweden, with high completeness of variables, e.g., 98% for educational level [Citation26]. Four references were matched individually to one survivor by sex, age, birth country (Sweden or outside), and educational level (N = 26,716).
Outcome measures
The first outcome was number of specialized outpatient physician visits and number of inpatient days, respectively, categorized according to the main diagnoses. The ICD-10 codes were used to identify and categorize the diagnoses into disease chapters (excluding visits and hospitalization related to pregnancy, childbirth, screening visits, etc. [Supporting Table 1]). This information was obtained from the National Patient Register, held by the National Board of Health and Welfare [Citation27]. This register became nationwide in 1987 for the inpatient part and 2001 for the outpatient part [Citation28]. The important variables including main diagnosis and personal identification number in the inpatient register have had 99% of coverage [Citation27,Citation28]. For the outpatient register, the coverage regarding main diagnoses was lower in the first few years, i.e., for 25–30% of recorded visits, main diagnoses were lacking, mainly regarding mental diagnoses [Citation28]. Thereafter, the coverage of important variables such as main diagnoses increased to 97% [Citation28]. In Sweden, healthcare and prescribed medication is highly subsidized for all residents.
Table 1. Characteristics of the study population.
The second outcome was mean numbers of combined sickness absence/disability pension net days. Information about start and end dates of sickness absence and disability pension spells was obtained from the MicroData for Analysis of the Social Insurance database (MiDAS) [Citation29] held by the Swedish Social Insurance Agency. This is an administrative, not clinical, register about paid benefits, meaning that its accuracy is very high. All Swedish residents >15 years with income from work or unemployment benefits can be granted sickness absence benefits in case of work incapacity due to disease or injury. Employers pay the first 14 d of a sickness absence spell, while the Social Insurance Agency provides benefits for sickness absence spells >14 d. In our study, only information about sickness absence spells >14 d was used.
All residents aged 19–64 years with long-term or permanent work incapacity due to disease or injury can be granted disability pension. Sickness absence benefits amount to 80% and disability pension to about 64% of lost income, up to a certain level. Sickness absence and disability pension can be granted for 25%, 50%, 75%, or 100% of ordinary work hours. Therefore, individuals can be on both partial sickness absence and disability pension simultaneously. Thus, net days of sickness absence and disability pension were used, calculated through multiplying the degrees with the number of days: e.g., 2 absence days at 50% equaled one net day.
Other covariates
Sociodemographic variables included sex, age, birth country, and educational level regarding 31 December the year before diagnosis date (). Cancer stage was classified into 0, I–IV, and missing, according to T, N, and M based either on clinical or histopathological examination registered in the Cancer Register [Citation30]. With a missing/‘X’ (assessment not possible) value at T and/or N and M, stage value was set to missing. If >1 entry of the same cancer type (colon or rectal cancer) diagnosis occurred in the register within 30 d, the most advanced staging was chosen. Charlson comorbidity index (CCI) [Citation31] was calculated based on in- and specialized outpatient healthcare during the 3 years before diagnosis date. Mental morbidity was defined as having had healthcare with ICD-10 codes of ‘F00-F99’ or ‘Z73’ or having bought any prescribed psychiatric medication (Anatomic Therapeutic Chemical codes: N05A, N05BA, N05BE01, N06A, N06AX12) during the 3 years before diagnosis date. Information on medication was obtained from the Prescribed Drug Register [Citation32] with information on all dispensed prescribed medicines, held by the National Board of Health and Welfare, who also keeps the Cause of Death Register (for information on death) [Citation33].
Statistical analyses
Survivors and references were followed from 2 years before (Y−2) inclusion at diagnosis date (T0) up to 5 years after (Y+5) (diagnosis date of matched survivors was also used as T0 for references).
In the analyses, individuals were censored from the year after they died, emigrated, or turned 65 (standard old-age pension age), whichever came first. In all analyses, the matched references of censored survivors were also excluded at the same time of the censoring of the index persons.
To assess healthcare use, outpatient visits and inpatient days with a main diagnosis of CRC within 30 d before diagnosis date were set to Y+1. Annual mean numbers of outpatient visits and inpatient days by ICD-10 chapter (Supplementary Table 1) were calculated for survivors and references for all 7 years.
Pre-diagnostic sickness absence was assessed by using sickness absence in Y−2. Therefore, the few people not living in Sweden in Y−2 were excluded from the sickness absence and disability pension analyses. Crude mean numbers of combined sickness absence and disability pension days by survivor subgroups in Y+3 and Y+5, respectively, were calculated with 95% confidence intervals (CIs). Further, we calculated adjusted mean numbers by adjusting mutually for all the covariates. Among these adjusted covariates, cancer-related covariates were adjusted for in the survivor cohort but not in the reference cohort.
Statistical analyses were performed with STATA version 14 (StataCorp, College Station, TX) . Level of significance was set at p < .05.
The project was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Results
Characteristics of the study population are in . The matching was adequate and only one of the other variables differed substantially between CRC survivors and references, namely pre-diagnostic comorbidity, which was much higher in CRC survivors (18% vs. 4%).
Secondary healthcare use
Specialized outpatient visits
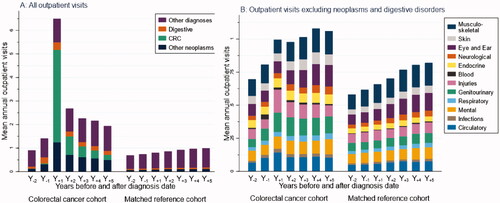
The mean number of outpatient visits in Y−2 was higher among CRC survivors compared to among references (0.91 vs. 0.70 visits) (). Post-diagnosis, the number peaked among survivors with 6.49 visits in in Y+1, then gradually decreased to 1.94 visits in Y+5 (1.00 visits among references). Among survivors’ outpatient visits in Y+1, CRC and other neoplasms accounted for a mean of 3.93 and 1.25 visits (0.22 and 0.49, respectively, in Y+5), while the mean number of outpatient visits also increased for digestive disorders (0.32 mean visits in Y+1; 0.17 in Y+5). Meanwhile, among ‘other diagnoses’ (), the most common ones in Y+1 were injuries (0.17 mean visits), genitourinary disorders (0.15 mean visits), and circulatory disorders (0.14 mean visits).
Figure 1. Healthcare use per yearly interval during 2 years before (Y−2) up to 5 years after (Y+5) diagnosis date for colorectal cancer (CRC) survivors and for their matched references (date of diagnosis CRC cohort; for matched reference group, the diagnosis date of the matched patient was used). (A) Mean number of outpatient visits (stacked) by CRC, other neoplasms, digestive disorders and other disorders. (B) Mean number of outpatient visits (stacked) by diagnostic ICD-10 chaptera, excluding neoplasms and digestive disorders. People not living in Sweden during the second year before diagnosis (Y−2), and those who died, emigrated, or turned 65 before the year of the corresponding analyses were excluded. Only the matched references of survivors included in the analyses were used. aDetails in Supplementary Table 1.
Inpatient days
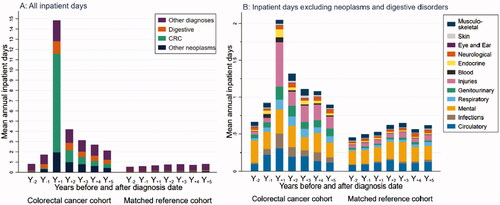
Survivors had higher mean numbers of inpatient days than references all 7 years (). In Y+1, mean inpatient days among survivors were 14.85 and decreased to 2.13 in Y+5 (references: 0.68 and 0.82, respectively). Among survivors in Y+1, 9.65 mean inpatient days were due to CRC, 1.94 due to other neoplasm diagnoses, and 1.21 due to digestive disorders (respective numbers for Y+5: 0.40, 0.43, and 0.39). The most common categories among ‘other diagnoses’ () in Y+1 were injuries (0.60 mean days), mental disorders (0.33 mean days), and circulatory disorders (0.30 mean days).
Figure 2. Healthcare use per yearly interval during 2 years before (Y−2) up to 5 years after (Y+5) diagnosis date for colorectal cancer (CRC) survivors and for the matched references (date of diagnosis for CRC cohort; for matched reference group, the diagnosis date of the matched patient was used). (A) Mean number of inpatient days (stacked) by CRC, other neoplasms, digestive disorders, and other disorders. (B) Mean number of inpatient days (stacked) by diagnostic ICD-10 chaptera, excluding neoplasms and digestive disorders. People not living in Sweden during the second year before diagnosis (Y−2), and those who died, emigrated, or turned 65 before the year of the corresponding analyses were excluded. Only the matched references of survivors included in the analyses were used. aDetails in Supplementary Table 1.
Sickness absence and disability pension net days
Among survivors, 40.3% in Y+3 and 35.0% in Y+5 had at least some sickness absence/disability pension days, compared to 23.3% and 23.5% among references.
Adjusted mean sickness absence/disability pension days/year among all CRC survivors decreased from 85 (95% CI 82–89) in Y+3 to 77 (95% CI 73–81) in Y+5 but were still higher than in the references (Y+3: 57 [95% CI 55–59]; Y+5: 54 [95% CI 51–56]) ().
Table 2. Crude and adjusted mean sickness absence and disability pension days in the study population during Y+3 and Y+5, and the relative risks (RRs) of sickness absence and disability pension days in colorectal cancer (CRC) survivors and matched references.
Further, adjusted mean sickness absence/disability pension days were highest among survivors with pre-diagnostic sickness absence >180 d (188 d in Y+5). Furthermore, in Y+5, high numbers of mean sickness absence/disability pension days were observed in stage IV survivors (83 d), those with pre-diagnostic mental morbidity (166 d), and CCI ≥2 (117 d), and only elementary education (129 d).
Overall, the distribution patterns of mean sickness absence/disability pension days within survivor subgroups were comparable to that of the references, however, at higher levels in all specific subgroups, with one exception of previous sickness absence days.
The categorical distribution of sickness absence/disability pension days was presented in Supplementary Table 2. In Supplementary Table 3, mean sickness absence and disability pension days in Y+3 and in Y+5 were presented by sickness absence and disability pension, respectively. Especially the number of disability pension days tended to be higher in the CRC survivors than in references. Exceptions to this were previous comorbidities and previous mental disorders.
Discussion
Compared with the matched references, the 6679 CRC survivors had both a continuously higher healthcare use and more sickness absence or disability pension days during the 5-year follow-up, though in a gradually declining manner. In Y+5, CRC survivors had more than double mean inpatient days and outpatient visits, respectively, than their references. Mean numbers of sickness absence/disability pension days were almost twice as high in survivors than in references in Y+3 and gradually decreased to 1.5 times higher in Y+5. It might be relevant to consider factors such as pre-diagnostic sickness absence/disability pension days, pre-diagnostic morbidity, immigrant background, and low educational level in future studies as well as when clinically planning support for CRC survivors.
Healthcare use
Currently, for the first time, we presented levels of specialized healthcare use among CRC survivors; also, by specific diagnoses. Our findings are complementary to previous studies on healthcare use of CRC survivors. Particularly, higher levels of primary healthcare contacts and referrals were previously shown among Dutch CRC survivors of all ages from 1-year pre-diagnosis to 6-year post-diagnosis [Citation14,Citation15]. A Danish study presented higher hospitalization risk of new somatic diseases in CRC survivors aged ≥40 years than in CRC-free references during five post-diagnosis years [Citation16]. In line with our findings, these studies showed higher risk of digestive, genitourinary, blood, endocrine, and mental diagnoses following CRC diagnosis [Citation14–16]. In contrast to the Danish study, we provided an overview of all specialized healthcare use, including hospitalization and regular CRC follow-up, and further showed the specific health needs of younger CRC survivors who are still part of the labor force as opposed to the above-mentioned studies that also included older patents.
Among survivors’ specialized healthcare use post-diagnosis due to cancer other than CRC, the most common diagnoses were secondary malignancies of respiratory and digestive organs. This is in line with our finding that 18% of survivors had a stage IV cancer already at diagnosis, that is, were diagnosed late in their cancer trajectory.
Notably, among the other diagnostic categories, injury was the most common diagnosis behind inpatient days and outpatient visits in Y+1, mainly attributable to complications of surgical and medical care (ICD-10 codes: T80–88). This finding is in line with another study [Citation34], which showed that CRC patients have higher risks for accidental puncture or rupture of wounds and infections during the diagnostic period. Thus, the long-term health impact of injuries related to cancer care in CRC survivors needs to be included in future studies.
Previously, a study including 34,662 CRC survivors in Sweden aged ≥18 years found a higher risk of future mental diagnoses based on inpatient and specialized outpatient visits, compared with CRC-free individuals [Citation35]. The risk was almost ten times higher during the first year post-diagnosis, after which it gradually decreased to the same level as the references’ after 10 years. The CRC survivors also had a higher use of psychiatric medication during 2 years post-diagnosis [Citation35].
Nevertheless, we showed that the level of specialized healthcare use with mental diagnoses remained comparable between survivors and references, both before and long-term after CRC diagnosis. Therefore, we suggest that even though CRC survivors may be afflicted by mental disorders, the impact is not profound enough to result in higher excess of specialized healthcare use with mental diagnoses.
The higher levels of specialized healthcare use are in line with the higher proportion of survivors having a pre-diagnostic comorbidity score ≥2 compared to references. Shared risk factors for certain diseases (cardiovascular, endocrine disorders) and CRC development [Citation36,Citation37] could explain the higher number of comorbidities among survivors. A U.S. report on CRC patients aged ≥66 found that survivors’ pre-diagnostic comorbidities have an important part in survival, especially in survivors with localized and regional cancer [Citation11]. These findings highlight the importance of health problems other than cancer during the survivorship phase.
Sickness absence and disability pension
Of high importance here is the finding that already in the third year after diagnosis date; the mean number of absence days was below 3 months. This is an important finding to inform patients about. Our findings of higher levels of sickness absence and disability pension even several years after CRC diagnosis than among references are in line with three previous studies [Citation19–21]. In this study, we added knowledge by presenting the extent of sickness absence/disability pension days by different factors, therefore, providing valuable information for survivors, healthcare practitioners, and employers. Furthermore, the use of matched references enabled us to assess the influence of CRC diagnosis and treatment on future sickness absence and disability pension. Mean sickness absence/disability pension days/year varied a great deal depending on survivors’ characteristics but were higher in all subgroups compared to in the references.
Besides showing that cancer-related factor, i.e., cancer stage was associated with higher post-diagnosis mean number of sickness absence/disability pension days, we also found that many risk factors existed pre-diagnosis, factors that might not be cancer-related. For example, consistent with other studies, pre-diagnostic sickness absence and other types of morbidity [Citation19–21,Citation38], lower educational level [Citation19,Citation20], and being born outside of Sweden (a risk factor not often studied) were strongly associated with higher mean number of post-diagnosis sickness absence/disability pension days. Survivors with such characteristics may more often have manual jobs and be at workplaces with less flexibility, which could be possible explanations for the outcomes [Citation39,Citation40]. All of these findings emphasize that the support for CRC survivors needs an integrated approach clinically, including also aspects of pre-morbid non-cancer-related characteristics, and early identification of patients at high risk of long-term sickness absence and/or disability pension.
Furthermore, as expected, and consistent with previous studies [Citation19–21,Citation38], sickness absence/disability pension days increased with cancer stage, possibly also due to longer and more aggressive treatments. Survivors aged 61–62 compared to ≤60 years when diagnosed had lower sickness absence/disability pension rates, possibly they took early old-age pension.
Strengths and limitations
A major strength was the use of high-quality data linked from several population-based registers [Citation27,Citation41], the longitudinal design covering 7 years, including all the CRC patients diagnosed during the period, allowing sub-group analyses. Through the high completeness of the registers, selection bias and loss of follow-ups were avoided [Citation42]. Further, the use of administrative data eliminated report bias through self-reports. Therefore, the results can be considered representative for Sweden and countries with similar social insurance and healthcare systems. Another strength was that people could be followed in relation to diagnosis date, rather than by calendar year. So far, studies on healthcare use of CRC survivors have focused on primary healthcare and mostly included CRC survivors ≥65 years [Citation12,Citation14,Citation15]. Our study depicts secondary healthcare use, therefore, focusing on more severe morbidity. There is no nationwide register of primary healthcare in Sweden thus this could not be included. This can be seen as both a strength and a limitation. The same can be said about not having information about shorter sickness absence spells. That the calculation of the CCI did not include primary healthcare data can be regarded as another limitation. However, using CCI derived from secondary healthcare records is shown at least as accurate as from primary healthcare records in predicting short- and long-term survival [Citation43]. Thus, the use of CCI only on secondary healthcare data may not have impacted the study results substantially. Another limitation is lack of specific information on the classification of cancer staging, synchronous tumors, cancer relapse, and treatment. This study serves as a first explorative investigation in this field and future studies with good quality data from CRC clinical register are highly needed. Further, it was possible that healthcare use may be underestimated in the study, due to limitations in coverage and validity in the National Patient Register, particularly the outpatient part in the first few study years. Thus, potential such and other misclassification bias may have occurred, however, that applied for both survivors and references.
Conclusion
CRC survivors of working-age had higher secondary healthcare use than their matched references both before and 5 years post-diagnosis. Notably, the higher healthcare use was not only due to cancers and digestive disorders, but also injuries, whereas no differences regarding mental healthcare use were found. Further, all subgroups of CRC survivors had higher levels of sickness absence/disability pension days than references, especially survivors with pre-diagnostic sickness absence, mental morbidity, comorbidities, lower educational level, born outside of Sweden, and advanced cancer stage. Nevertheless, the number of sickness absence/disability pension days 3- and 5-year post diagnosis was less than three months. Our findings show that the survivors’ situation pre-diagnosis plays an important role for future work capacity, suggesting that these factors could be used early on to identify survivors who might benefit most from rehabilitation and return-to-work measures.
Overall, these findings emphasize the importance of focusing on survivors’ non-cancer-related needs during the survivorship, especially when planning return-to-work measures.
ionc_a_1974551_sm3762.docx
Download MS Word (57.8 KB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Holleczek B, Rossi S, Domenic A, et al. On-going improvement and persistent differences in the survival for patients with Colon and rectum cancer across Europe 1999–2007 – results from the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2158–2168.
- Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet. 2019;4(7):511–518.
- Söderman M, Wennman-Larsen A, Alexanderson K, et al. Oncologists’ experiences of and prerequisites for sickness certification tasks: a nationwide questionnaire study. Eur J Cancer Care (Engl). 2021;30(4):e13414.
- Bränström R, Arrelöv B, Gustavsson C, et al. Sickness certification at oncology clinics: perceived problems, support, need for education and reasons for certifying unnecessarily long sickness absences. Eur J Cancer Care (Engl). 2014;23(1):89–97.
- Ljungquist T, Hinas E, Nilsson GH, et al. Problems with sickness certification tasks: experiences from physicians in different clinical settings. A cross-sectional nationwide study in Sweden. BMC Health Serv Res. 2015;15(1):321.
- Söderman M. Healthcare professionals’ work with sickness absence – with focus on oncology. Solna: Karolinska Institutet; 2020.
- Alfano CM, Kent EE, Norton WE, et al. Recommendations for research and practice to improve work outcomes among cancer Survivors. J Natl Cancer Inst. 2018;110(10):1041–1047.
- Lloyd S, Baraghoshi D, Tao R, et al. Mental health disorders are more common in colorectal cancer survivors and associated with decreased overall survival. Am J Clin Oncol. 2019;42(4):355–362.
- Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw. 2009;7(8):883–893.
- Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314.
- Steele CB, Townsend JS, Tai E, et al. Physician visits and preventive care among Asian American and pacific islander long-term survivors of colorectal cancer, USA, 1996–2006. J Cancer Surviv. 2014;8(1):70–79.
- Claire FS, Kevin DF, Robert JH, et al. Quality of care for comorbid conditions during the transition to survivorship: differences between cancer survivors and noncancer controls. J Clin Oncol. 2013;31(9):1140–1148.
- Brandenbarg D, Roorda C, Groenhof F, et al. Primary healthcare use during follow-up after curative treatment for colorectal cancer. Eur J Cancer Care. 2017;26(3):e12581–n/a.
- Brandenbarg D, Roorda C, Groenhof F, et al. Increased primary health care use in the first year after colorectal cancer diagnosis. Scand J Prim Health Care. 2014;32(2):55–61.
- Kjaer TK, Andersen EAW, Winther JF, et al. Long-term somatic disease risk in adult danish cancer survivors. JAMA Oncol. 2019;5(4):537–545.
- Earle CC, Chretien Y, Morris C, et al. Employment among survivors of lung cancer and colorectal cancer. J Clin Oncol. 2010;28(10):1700–1705.
- Gordon L, Lynch BM, Newman B. Transitions in work participation after a diagnosis of colorectal cancer. Aust N Z J Public Health. 2008;32(6):569–574.
- Chen L, Glimelius I, Neovius M, et al. Work loss duration and predictors following rectal cancer treatment among patients with and without prediagnostic work loss. Cancer Epidemiol Biomarkers Prev. 2016;25(6):987–994.
- Hauglann BK, Saltytė Benth J, Fosså SD, et al. A controlled cohort study of sickness absence and disability pension in colorectal cancer survivors. Acta Oncol. 2014;53(6):735–743.
- Chen L, Glimelius I, Neovius M, et al. Risk of disability pension in patients following rectal cancer treatment and surgery. Br J Surg. 2015;102(11):1426–1432.
- Blinder VS, Gany FM. Impact of cancer on employment. J Clin Oncol. 2020;38(4):302–309.
- Ludvigsson J, Otterblad-Olausson P, Pettersson B, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- National Board of Health and Welfare. Bortfall och kvalitet om cancerregistret (Loss and quality in Swedish Cancer Register, in Swedish). 2019. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/cancerregistret/bortfall-och-kvalitet/
- WHO. International classification of diseases. World Health Organization; 2020. [updated 2020-03-04 16:46:40;19 May 2020]. Available from: https://www.who.int/classifications/icd/icdonlineversions/en/
- Ludvigsson JF, Svedberg P, Olen O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research [review. Eur J Epidemiol. 2019;34(4):423–437.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- National Board of Health and Welfare. Bortfall och kvalitet i patientregistret (Loss and quality in the patient register, in Swedish). 2020. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/patientregistret/bortfall-och-kvalitet/
- Österlund N. MiDAS Sjukpenning och Rehabiliteringspenning Version 1.02 [The MiDAS register. Sickness absence benefits] (in Swedish). Stockholm: Swedish Social Insurance Agency; 2011.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2010.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Wettermark B, Hammar N, Michaelfored C, et al. The new Swedish prescribed drug Register-opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- Brooke HL, Talback M, Hornblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Shen Q, Lu D, Schelin MEC, et al. Injuries before and after diagnosis of cancer: nationwide register based study. BMJ. 2016;354:i4218.
- Lu D, Andersson TML, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: a nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2(9):1188–1196.
- Duarte CW, Lindner V, Francis SA, et al. Visualization of cancer and cardiovascular disease co-occurrence with network methods. JCO Clin Cancer Inform. 2017;1:1–12.
- Jiang Y, Ben Q, Shen H, et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26(11):863–876.
- Carlsen K, Harling H, Pedersen J, et al. The transition between work, sickness absence and pension in a cohort of Danish colorectal cancer survivors. BMJ Open. 2013;3(2):e002259.
- Dorland HF, Abma FI, Roelen CAM, et al. Factors influencing work functioning after cancer diagnosis: a focus group study with cancer survivors and occupational health professionals. Support Care Cancer. 2016;24(1):261–266.
- Taskila-Brandt T, Martikainen R, Virtanen SV, et al. The impact of education and occupation on the employment status of cancer survivors. Eur J Cancer. 2004;40(16):2488–2493.
- Swedish Social Insurance Agency. Social insurance in figures 2017. Stockholm: Swedish Social Insurance Agency; 2017.
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33.
- Crooks CJ, West J, Card TR. A comparison of the recording of comorbidity in primary and secondary care by using the Charlson index to predict short-term and long-term survival in a routine linked data cohort. BMJ Open. 2015;5(6):e007974.
