Abstract
Background
Neoadjuvant chemotherapy is often used prior to surgical resection for oesophageal adenocarcinoma but remains ineffective in a high proportion of patients. The histological Mandard tumour regression grade is used to determine chemoresponse but is not available at the time of treatment decision-making. The aim of this cohort study was to identify factors that predict chemotherapy response prior to surgery.
Methods
A prospectively collected database of patients undergoing surgical resection for oesophageal adenocarcinoma from a high-volume UK institution was used. Patients were subcategorised using pathological tumour response into ‘responders’ (Mandard grade 1–3) and ‘non-responders’ (Mandard grade 4 and 5). Multivariable logistic regression analysis was performed to calculate crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) for responder status adjusting for a variety of parameters. Receiver operating characteristic (ROC) curves were calculated.
Results
Among 315 patients included, 102 (32%) were responders and 213 (68%) non-responders. A decrease in radiological tumour volume (OR 1.92 95%CI 1.02–3.62; p = 0.05), a ‘partial response’ RECIST score (OR 7.16 95%CI 1.49–34.36; p = 0.01), a clinically improved dysphagia score (OR 2.79 95%CI 1.05–7.04; p = 0.04) and lymphovascular invasion (OR 0.06 95%CI 0.02–0.13; p = 0.000) influenced responder status. ROC curve analysis for responder status utilising all available parameters had an area under the curve (AUC) of 0.86.
Conclusion
This study has highlighted the potential for using pre-defined factors to identify those patients who have responded to neoadjuvant chemotherapy, prior to surgical resection, potentially facilitating a more individualised therapeutic approach.
Introduction
Despite advances in treatment strategies and improvements in overall survival rates, oesophageal cancer remains an important cause of cancer-related deaths worldwide [Citation1]. Most patients present with locally advanced or metastatic disease [Citation2,Citation3] and as a result, only a minority of patients are suitable for potentially curative resection.
UK practice has been influenced by a number of large randomised clinical trials which have demonstrated a significant survival benefit for patients treated with neoadjuvant therapy when compared to surgery alone [Citation4–8]. The potential benefits of preoperative therapy include downstaging the primary tumour, improving rates of complete surgical resection (R0) and treating occult micro-metastases [Citation9]. The National Oesophago-Gastric Cancer Audit (NOGCA) has demonstrated that most patients in the UK are now offered neoadjuvant chemotherapy, rather than neoadjuvant chemoradiotherapy; however, trials comparing the two treatment approaches are ongoing [Citation10,Citation11].
One of the main challenges of neoadjuvant therapy is determining, as early as possible, which patients are not responding to treatment so as to avoid the significant toxicity of ineffectual chemotherapy. Methods of assessing response include symptomatic, endoscopic and radiological evaluation, but these are generally performed after the completion of treatment and have thus not facilitated a tailored management approach [Citation12]. The histological assessment of the primary tumour, using the Mandard tumour regression grading system [Citation13] provides a definitive evaluation of response; however, this is only available after surgical resection and cannot be used to prospectively guide the decision-making process.
The aim of this study was to evaluate the association between clinical and radiological factors in relation to pathological response to neoadjuvant chemotherapy and to determine if a combination of these factors could be used to predict response.
Methods
Data source
This was a single centre cohort study of patients with oesophageal adenocarcinoma, including gastro-oesophageal junctional adenocarcinoma, from a large, high-volume, tertiary referral centre in the UK. St Thomas’ Hospital in London accepts oesophago-gastric cancer referrals from London and the South East of England and provides specialist surgical and oncological care. This study used a prospectively maintained database of all patients undergoing oesophagogastrectomy for cancer.
Study cohort
From the hospital-based operative database, all patients who underwent surgical resection following chemotherapy for oesophageal adenocarcinoma between January 2008 and December 2016 were identified. All patients were discussed in a specialist oesophagogastric cancer multi-disciplinary team meeting and had at least 12 months of complete follow-up data. Transthoracic or transhiatal oesophageal resections were performed, dictated by tumour location, at the discretion of the individual surgeon. Open and minimally invasive techniques were included. All resection specimens were examined by specialist gastrointestinal pathologists and all patients were allocated a tumour regression grade according to the Mandard classification [Citation13]. Patients with a tumour regression grade of 1–3 were considered to have had a response to neoadjuvant chemotherapy (‘responders’) and those with a tumour regression grade of 4–5 were considered not to have responded (‘non-responders’).
Radiological and clinical parameters
A variety of parameters were assessed at baseline and following completion of neoadjuvant chemotherapy. All patients had a computed-tomography (CT) scan performed as part of their initial staging investigations. A CT scan was then repeated after neoadjuvant treatment, prior to surgical resection. Radiological factors assessed included CT staging using the 7th edition of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging system [Citation14], a RECIST (Response Evaluation Criteria in Small Tumours) 1.0 score [Citation15] allocated by a specialist consultant gastrointestinal radiologist in addition to measurements of tumour height, width and volume. The volume of the primary tumour was calculated by measuring the longest axial and craniocaudal diameter of the tumour using the axial source images and sagittal reformats. The radiological tumour volume (RTV) was then derived using a validated conical volume formula [Citation16]; v= (ab2)π/6 where v = volume, a = maximum length of tumour and b = maximum width of tumour. This validated formula assumes that the estimated RTV is determined as the sum of two truncated cones [Citation16]. All volume calculations were performed by two dedicated senior radiologists with experience in gastrointestinal oncology. A previous study from our institution demonstrated very high levels of correlation between the volume formula, CT volume software (open source, MAC-based DICOM Viewer, OsiriX 3.9) [Citation17] and pathological tumour volume [Citation18].
Clinical factors assessed included patient weight and dysphagia scores retrieved both at baseline and after completion of neoadjuvant chemotherapy. The dysphagia score was measured using a validated pre-operative cancer-specific questionnaire (EORTC QLQ-OG25) [Citation19]. Length of in-hospital stay, 30-day mortality and recurrence patterns were also recorded. The information was recorded prospectively by dedicated dieticians and oesophagogastric cancer nurse specialists during routine clinical assessments.
Neoadjuvant chemotherapy
All patients received platinum and fluoropyrimidine-based chemotherapy regimens including CF (cisplatin and 5-fluorouracil), ECF (epirubicin, cisplatin, 5-fluorouracil), ECX (epirubicin, cisplatin and capecitabine) or EOX (epirubicin, oxaliplatin and capecitabine) with the majority of patients completing the standard two to four cycles as per randomised trial evidence available at the time of treatment. This study was conducted prior to the introduction of the FLOT (5-fluorouracil, leucovorin, oxaliplatin and taxotere) regimen.
Outcomes
Pre-defined radiological and clinical parameters assessed included tumour location, decrease in tumour height and width, >50% decrease in tumour volume, decrease in TNM staging, RECIST scoring, change in dysphagia score and change in patient’s weight. The histological Mandard tumour regression grading classification system was used to categorise patients as ‘responders’ (grade 1–3) or ‘non-responders’ (grade 4–5). Survival was defined as days from the date of surgical resection until the date of death. Time to recurrence was calculated from the date of surgical resection until disease recurrence, defined as either histopathological or definitive radiological evidence of local recurrence, systemic recurrence or both. In the absence of recurrence, survival was calculated to the last confirmed attendance at a hospital or general practitioner clinic.
Statistical analysis
Basic demographic, surgical and oncological data were evaluated using descriptive statistics. Logistic regression analysis (crude and adjusted) was used to calculate odds ratios (OR) with 95% confidence intervals (CI) to model the likelihood of different variables being associated with responder versus non-responder status. Non-responder status was the reference group for all analyses. Variables included in the multivariable model were age, sex, American Society of Anaesthesiologists (ASA) grade (2 or 3), tumour location (oesophageal, Siewert 1 or Siewert 2), decrease in tumour height (cm- yes or no), decrease in tumour width (cm- yes or no), decrease in CT volume (>50%) (cm3- yes or no), decrease in tumour T-stage (radiological), RECIST score (stable disease, partial response or progressive disease), neoadjuvant chemotherapy regimen (CF, ECF, ECX, EOX or other), lymphovascular invasion (yes or no), presence of Barrett’s oesophagus (yes or no), presence of signet ring cells (yes or no), change in dysphagia score (no change, improved or deteriorated) and change in patient weight (no change, decrease or increase).
A Cox proportional hazards model for survival analysis was also performed. Outcome measures were time to death, time to recurrence or time to last hospital or general practitioner follow-up appointment. This model was adjusted for the same parameters and categorisations as in the logistic regression analysis presented above. The Kaplan–Meier method was used to calculate and compare crude survival between responders and non-responders.
Receiver operating characteristic curve analysis was performed to assess the likelihood of predicting responder status by combining statistically significant radiological and clinical variables. The area under the curve (AUC) was used for discrimination with an AUC of 1 corresponding to perfect discrimination and an AUC of 0.5 suggesting no discrimination.
All statistical analyses were performed using STATA version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, USA: StataCorp LLC) and a p value <0.05 was used to define statistical significance for all outcomes.
Results
Study cohort
Patient demographics, oncological and surgical characteristics according to response to neoadjuvant chemotherapy are summarised in . The database included 315 consecutive patients with oesophageal adenocarcinoma who had completed a course of neoadjuvant chemotherapy prior to surgical resection. Of these patients, 102 (32.4%) were ‘responders’ and 213 (67.6%) were ‘non-responders’.
Table 1. Demographics, oncological and surgical characteristics according to response to neoadjuvant chemotherapy in patients who underwent surgery for oesophageal adenocarcinoma.
The majority of patients were male (82.4%). In the responder patient group, the majority of patients had an R0 resection (71 patients, 69.6%), whereas in the non-responder group more patients had an R1 resection (121 patients, 56.8%) according to the Royal College of Pathologists criteria of tumour at, or within 1 mm, of the cut margin [Citation20]. Lymphovascular invasion was only present in 26 (25.5%) patients in the responder group and in 159 (74.7%) patients in the non-responder group.
There were two (0.9%) in-hospital deaths, one of which was within 30 days of surgical resection. These deaths occurred in the non-responder patient group. The median length of stay after surgical resection was 12 days in both patient groups. Tumour recurrence was more common in the non-responder group (107 patients, 50.2% vs. 24 patients, 23.5%) and it was most often systemic in nature. Median time to recurrence and death were both shorter in the non-responder group.
Radiological and clinical parameters
Radiological and clinical data collected before and after completion of neoadjuvant chemotherapy are summarised in Supplementary Tables 1 and 2. Presence of dysphagia and weight loss at the time of diagnosis was similar across the two patient groups. Responders were more likely to demonstrate a partial response RECIST score (p = 0.01), a greater than 50% decrease in CT tumour volume (p = 0.05) and an improvement in swallowing (p = 0.04) than non-responders.
Logistic regression
Results for crude and adjusted logistic regression analyses including are presented in . A more than 50% decrease in CT tumour volume (OR 1.92 95%CI 1.02–3.62 p = 0.05), a partial response RECIST score (OR 7.16 95%CI 1.49–34.36 p = 0.01) and a clinically improved dysphagia score (OR 2.79 95%CI 1.05–7.40 p = 0.04) were all associated with responder status. A decrease in tumour width (cm) also favoured responder status (OR 0.30 95%CI 0.08–1.13 p = 0.07). Lymphovascular invasion was strongly associated with non-responder status (OR 0.06 95%CI 0.02–0.13 p = 0.000).
Table 2. Crude and adjusted odds ratios (OR) with 95% confidence intervals (CI) and p values for key parameters included in the logistic regression model in patients who underwent surgery for oesophageal adenocarcinoma.
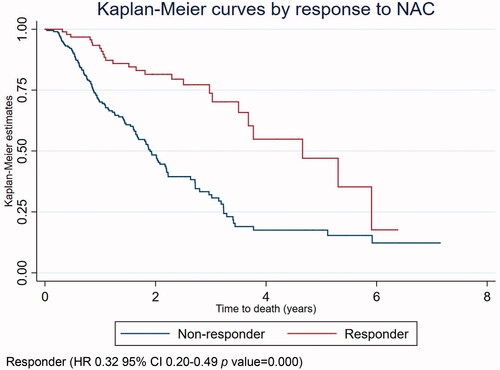
Kaplan–Meier survival curve
A crude Kaplan–Meier survival curve for overall survival in responders and non-responders is presented in .
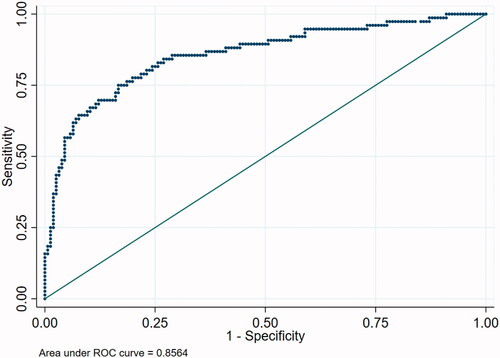
Receiver operating characteristic (ROC) curves
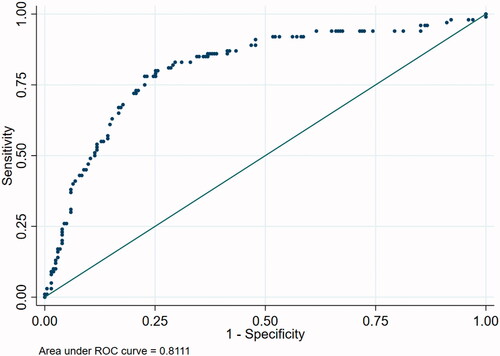
ROC curve analysis was performed using the whole logistic regression model and for different combinations of parameters. represents ROC curve analysis for responder status including all the parameters used in the logistic regression model. The AUC was 0.86. A simplified 6-point model including age, sex, RECIST score, change in CT volume, change in weight and change in dysphagia score was calculated (Supplementary Figure 1) with an AUC of 0.64. A 6-point model including age, sex, RECIST score, change in CT volume, change in dysphagia score and lymphovascular invasion () provided an AUC of 0.81.
Discussion
This study demonstrated that a number of factors, available prior to surgical resection, were associated with responder status. These included an improvement in dysphagia, a reduction in tumour volume and a positive response on RECIST scoring. Lymphovascular invasion was found to be strongly associated with non-responder status, a novel finding. Taken together, these factors were able to predict, with reasonable accuracy, those patients who were benefitting from neoadjuvant chemotherapy. As in many previous studies, responders to neoadjuvant chemotherapy demonstrated increased overall survival. Early identification of patients who are not responding to neoadjuvant treatment is an ongoing challenge in oesophageal adenocarcinoma as well as avoiding the significant side effects experienced with ineffectual chemotherapy. There is some evidence that persisting with chemotherapy in the non-responder group may lead to worse survival than surgery alone [Citation21].
There are some methodological constraints in the present study which merit consideration. This was a large cohort study, from a high-volume centre, of patients having neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma with long-term follow-up. Although this offers a realistic reflection of current practice, it remains impossible to eliminate bias in studies of this kind, despite adjustments for confounding factors. Any patients who were treated with neoadjuvant chemotherapy but did not proceed to surgical resection were excluded. Accurately predicting chemoresponse remains challenging and currently no effective biomarkers of response have been identified. In this study, the histological Mandard tumour regression grade was used to define response to neoadjuvant chemotherapy. This has been demonstrated to be prognostic in oesophageal cancer patients treated by neoadjuvant chemotherapy. However, this categorical score remains imperfect in identifying all true responders as it only assesses the primary tumour and because the mechanisms of chemotherapy response at a cellular level are poorly understood. Tumour down-staging on imaging [Citation21,Citation22] and lymph node regression [Citation23] is increasingly used to provide important supplementary information that can more accurately identify patients who are benefitting from neoadjuvant chemotherapy. This has been highlighted in a recent consensus report which proposed a new 4-tiered tumour regression grade system for assessing the primary tumour, combined with a 3-tiered system for grading therapeutic response in metastatic lymph nodes for patients with oesophageal and junctional adenocarcinoma [Citation24]. Although the included analysis would still be applicable using this new combined system in terms of primary tumour assessment, this is clearly an area where further research is required. Although it is logical to classify patients as either responders or non-responders, determining which group those patients with a Mandard grade of 3 should be allocated remains contentious [Citation25].
In the present study, an improved dysphagia score was associated with ‘responder’ status, which is in agreement with previous studies. It is a simple measure that can be identified through a routine clinical history. An improvement in swallowing after neoadjuvant chemotherapy has been shown to be a good indicator of radiological response; however, there is currently limited evidence to support using it as a valid predictive measure of pathological response [Citation2]. The present study would suggest otherwise, and it remains an important factor for the patient, which is easy to assess alongside other more specific radiological parameters.
A number of radiological factors were found in this study to be associated with ‘responder’ status; a greater than 50% decrease in CT tumour volume, a partial response RECIST score and a decrease in tumour width all favoured ‘responder’ status. These parameters are available prior to surgical resection and could potentially be assessed at specific time points throughout the duration of treatment, to tailor the ongoing treatment pathway to the individual patient. In the present study, a CT scan was performed after completion of three cycles of neoadjuvant chemotherapy, although it is acknowledged that earlier identification of non-responders would be preferable.
Numerous studies have assessed the value of positron emission tomography-computed tomography (PET-CT) as a predictor of chemotherapy response in oesophageal adenocarcinoma [Citation26–28]. There is agreement across these studies that the standardised uptake value (SUV) reduction in clinical responders is significantly higher than that of the non-responders and that this reduction may correlate with pathological response [Citation29]. In the MUNICON trials [Citation30,Citation31], the feasibility of a PET-guided treatment pathway, performed after the second cycle of chemotherapy, was demonstrated, although there was a failure to reach the primary endpoint of increasing the R0 resection rate in non-responders. Identifying non-responders early in the treatment pathway remains appealing because these patients could be considered for alternative chemotherapy regimens, surgery at an earlier stage (MUNICON I) [Citation30] or be diverted to chemoradiotherapy as was performed in the MUNICON II trial [Citation31]. More recently, further studies have attempted to use PET-CT to adjust therapy regimens in non-responders to chemoradiotherapy; however, so far no effect on survival has been observed [Citation32,Citation33]. Undoubtably, the addition of metabolic markers of response to the present model would be an interesting area for future study.
In this study, lymphovascular invasion was associated with non-responder status. Although lymphovascular invasion is not standardly available prior to surgical resection, it can be identified in patients with early stage tumours, from endoscopic mucosal resection or dissection specimens. There have also been recent studies, in other cancer types, which suggest that accurate prediction of lymphovascular invasion prior to surgery can be achieved using a variety of different techniques. One study demonstrated the use of pre-operative CT in predicting lymphovascular invasion in early rectal cancer by measuring the diameter of the superior haemorrhoidal vein [Citation34]. The prediction of lymphovascular invasion in breast cancer has been demonstrated using magnetic-resonance imaging and specifically the tumour apparent diffusion coefficient [Citation35]. More recently, another study demonstrated that a specific candidate gene can be used as a potential biomarker for predicting lymphovascular invasion in endometrial cancer [Citation36]. These encouraging results highlight the potential for future work where accurate prediction of lymphovascular invasion may have a role alongside other factors in determining response to neoadjuvant chemotherapy in oesophageal adenocarcinoma.
The presence of signet ring cells and microsatellite instability has also been associated with chemo-resistance in other studies [Citation37] and molecular profiling upfront is likely to become increasingly important. Interestingly, the presence of signet ring cells was not associated with non-responder status in the present study, which is in contrast to previous work; however, this would again be an interesting area for future study.
ROC analysis was used to create predictive models for response to neoadjuvant chemotherapy. When all the available parameters were included, the area under the curve was high. When the model was simplified to only six factors that were available prior to surgery, some discrimination was observed, albeit weaker. The inclusion of lymphovascular invasion significantly improved the performance of the predictive model, highlighting its value in chemotherapy response prediction. This novel finding should be a focus of future work.
In conclusion, this study has highlighted the potential for using a variety of parameters available prior to surgical resection of oesophageal adenocarcinoma, to identify those patients who respond to neoadjuvant chemotherapy, allowing for a more individualised approach. By identifying those patients who are non-responders, the treatment pathway for these patients may in future be tailored accordingly, thus avoiding the significant side effects associated with neoadjuvant chemotherapy.
Previous communication
AUGIS 22nd Annual Scientific Meeting, 25–27 September 2019, Liverpool, UK.
Bott R, Zylstra J, McEwan R, et al. AUGIS abstracts 2019 UGIC P80 – predicting pathological response to neoadjuvant chemotherapy in patients with oesophageal adenocarcinoma. Br J Surg. 2019;106(S7):88.
Supplemental Material
Download PDF (329.2 KB)Supplemental Material
Download MS Word (85.4 KB)Supplemental Material
Download MS Word (16.5 KB)Supplemental Material
Download MS Word (18.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Forshaw MJ, Gossage JA, Chrystal K, et al. Symptomatic responses to neoadjuvant chemotherapy for carcinoma of the oesophagus and oesophagogastric junction: are they worth measuring? Clin Oncol. 2006;18(4):345–350.
- Papaxoinis G, Kamposioras K, Weaver JMJ, et al. The role of continuing perioperative chemotherapy post surgery in patients with esophageal or gastroesophageal junction adenocarcinoma: a multicenter cohort study. J Gastrointest Surg. 2019;23(9):1729–1741.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067.
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084.
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO. Lancet Oncol. 2016;17(12):1697–1708.
- Urschel JD, Vasan H. A Meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–543.
- Hoeppner J, Lordick F, Brunner T, et al. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16:503.
- Reynolds JV, Preston SR, O'Neill B, et al. ICORG 10-14: NEOadjuvant trial in adenocarcinoma of the oEsophagus and oesophagoGastric junction international study (Neo-AEGIS). BMC Cancer. 2017;17(1):401.
- Forshaw MJ, Gossage JA, Mason RC. Neoadjuvant chemotherapy for oesophageal cancer: the need for accurate response prediction and evaluation. Surgeon. 2005;3(6):373–382.
- Mandard A‐M, Dalibard F, Mandard J, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686.
- Rusch V, Rice T, Crowley J, et al. The seventh edition of the American Joint Committee on cancer/international union against cancer staging manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg. 2010;139(4):819–821.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216.
- Créhange G, Bosset M, Fabrice L, et al. Tumor volume as outcome determinant in patients treated with chemoradiation for locally advanced esophageal cancer. Am J Clin Oncol Cancer Clin Trials. 2006;29(6):583–587.
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–216.
- Tullie LGC, Sohn HM, Zylstra J, et al. A role for tumor volume assessment in resectable esophageal cancer. Ann Surg Oncol. 2016;23(9):3063–3070.
- Lagergren P, Fayers P, Conroy T, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43(14):2066–2073.
- The Royal College of Pathologists. Dataset for the histopathological reporting of oesophageal carcinoma (2nd edition). Document G006. 2007. Available from: https://www.rcpath.org/uploads/assets/f8b1ea3d-5529-4f85-984c8d4d8556e0b7/068e9093-0aea-4316-bdd49771564784b9/g006-dataset-for-histopathological-reporting-of-oesophageal-and-gastric-carcinoma.pdf.
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32(27):2983–2990.
- Knight WRC, Baker CR, Griffin N, et al. Does a high mandard score really define a poor response to chemotherapy in oesophageal adenocarcinoma? Br J Cancer. 2021;124(10):1653–1660.
- Davies AR, Myoteri D, Zylstra J, et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br J Surg. 2018;105(12):1639–1649.
- Saliba G, Detlefsen S, Carneiro F, et al. Tumor regression grading after neoadjuvant treatment of esophageal and gastroesophageal junction adenocarcinoma: results of an international Delphi consensus survey. Hum Pathol. 2021;108:60–67.
- Saunders JH, Bowman CR, Reece-Smith AM, et al. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J Surg Oncol. 2017;115(7):821–829.
- Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19(12):3058–3065.
- Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4962–4968.
- Wieder HA, Ott K, Lordick F, et al. Prediction of tumor response by FDG-PET: comparison of the accuracy of single and sequential studies in patients with adenocarcinomas of the esophagogastric junction. Eur J Nucl Med Mol Imaging. 2007;34(12):1925–1932.
- Harada K, Mizrak Kaya D, Lopez A, et al. Personalized therapy based on image for esophageal or gastroesophageal junction adenocarcinoma. Ann Transl Med. 2018;6(4):80.
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8(9):797–805.
- Zum Büschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med. 2011;52(8):1189–1196.
- Ku GY, Kriplani A, Janjigian YY, et al. Change in chemotherapy during concurrent radiation followed by surgery after a suboptimal positron emission tomography response to induction chemotherapy improves outcomes for locally advanced esophageal adenocarcinoma. Cancer. 2016;122(13):2083–2090.
- Greally M, Chou JF, Molena D, et al. Positron-emission tomography scan–directed chemoradiation for esophageal squamous cell carcinoma: no benefit for a change in chemotherapy in positron-emission tomography nonresponders. J Thorac Oncol. 2019;14(3):540–546.
- Wu CC, Lee RC, Chang CY. Prediction of lymphovascular invasion in rectal cancer by preoperative CT. Am J Roentgenol. 2013;201(5):985–992.
- Igarashi T, Furube H, Ashida H, et al. Breast MRI for prediction of lymphovascular invasion in breast cancer patients with clinically negative axillary lymph nodes. Eur J Radiol. 2018;107:111–118.
- Watanabe T, Honma R, Kojima M, et al. Prediction of lymphovascular space invasion in endometrial cancer using the 55-gene signature selected by DNA microarray analysis. PLOS One. 2019;14:1–10.
- Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol. 2016;7(5):771–788.