Abstract
Background
Combination therapy with BRAF and MEK inhibitors (BRAF/MEKi) has significantly improved the outcome for patients with BRAF-mutated melanoma. A reduction in left ventricular ejection fraction (LVEF) is a known side effect during treatment with BRAF/MEKi. This study aimed to analyze sequential multigated acquisition (MUGA) scans for the evaluation of LVEF and provide real-world data on cardiotoxicity induced by BRAF/MEKi in advanced melanoma.
Methods
All patients with advanced melanoma treated with dabrafenib and trametinib at Herlev and Gentofte Hospital, Denmark, between March 2015 and September 2019, were included retrospectively. MUGA scans performed at baseline and every three months during treatment were analyzed. Cardiotoxicity was defined as a decline of ≥10 percentage point (pp) to an LVEF <50% (major cardiotoxicity) or a decline in LVEF of ≥15 pp but remaining >50% (minor cardiotoxicity).
Results
A total of 139 patients were included. Forty-six patients (33%) met our criteria for cardiotoxicity; 31 patients (22%) experienced minor cardiotoxicity and 15 patients (11%) experienced major cardiotoxicity. Median time to decline in LVEF was 94 days, and all clinically significant declines in LVEF occurred before evaluation at six months. Reversibility of LVEF was seen in 80% of patients, three patients were not evaluable for reversibility. A low left ventricular peak emptying rate adjusted for heart rate (LVPERadj) at baseline was found a potential risk factor for the development of major cardiotoxicity (RR = 0.159, p = 0.001).
Conclusion
A decline in LVEF is common for patients with advanced melanoma treated with BRAF/MEKi but rarely clinically significant. No significant decline in LVEF was observed after evaluation at six months, therefore routine monitoring of LVEF might be stopped after six to nine months of BRAF/MEKi therapy. A low LVPERadj might be a risk factor for the development of cardiotoxicity and is suggested for further investigation.
Background
Melanoma is an aggressive form of skin cancer, causing 55,500 cancer-related deaths annually worldwide, with an increasing incidence [Citation1,Citation2]. The most frequent mutation in melanoma is the BRAF mutation, present in about 50% of melanomas [Citation2,Citation3]. BRAF can be targeted with selective BRAF inhibitors (BRAFi). MEK (MEK1, MEK2) is a kinase downstream of RAF and is also used as a target with MEK inhibitors (MEKi). In large phase 3 clinical trials, the combination of BRAFi and MEKi has shown improved progression-free survival (PFS) and overall survival (OS) compared to BRAFi alone and previously used chemotherapies [Citation4–11].
Several studies have reported a risk of decline in left ventricular ejection fraction (LVEF) during treatment with BRAF/MEKi [Citation5–8,Citation10,Citation12–15]. MEK is not a mutated oncogene, and therefore inhibition may lead to affection also in normal tissues, e.g., the heart. MEK, and its substrate ERK are part of a group of pro-survival protein kinases that mediate cardioprotection, and inhibition of this pathway may alter cardiac protection [Citation15–18]. In cardiomyocytes, ERK is activated in response to several types of stress stimuli, and the cardiotoxic effect of MEKi might be mediated through suppression of ERK [Citation15,Citation19].
Although considered relatively rare, a decline in LVEF is recognized as a potentially severe adverse event during treatment with BRAF/MEKi and may lead to treatment interruption or discontinuation [Citation20–22]. Continuous monitoring of LVEF can assist in clinical decision making such as dose alterations or treatment discontinuation and, in case of a significant reduction in LVEF, help evaluate the need for initiation of heart failure medication. Cardiac dysfunction can be assessed by several methods, including echocardiography and multigated acquisition (MUGA) scans.
In this study, we analyzed sequential MUGA scans to investigate the extent and characteristics of cardiotoxicity in metastatic melanoma patients treated with BRAF/MEKi outside of clinical trials. We identified changes in ventricular volumes related to a reduction in LVEF and assessed the meantime to the affection of LVEF. Also, potential risk factors for the development of cardiotoxicity were investigated.
Methods
Study population
The Danish Metastatic Melanoma Database (DAMMED) contains information on all Danish melanoma patients receiving systemic antineoplastic therapy [Citation23]. The database was used to identify all patients receiving dabrafenib/trametinib at the Department of Oncology, Herlev and Gentofte University Hospital, Denmark, since the approval of MEKi in 2015 until data cutoff 1 September 2019. All patients registered in DAMMED have signed informed consent. The registry and consent form have received legal approval from the Danish Data Protection Agency (18/47885) and the Danish Patient Safety Authority (3-3013-1688/1/). The use of retrospective data until September 2016 was also approved, although no written consent was obtained before that date.
Inclusion criteria were diagnosis of stage III-IV unresectable melanoma, treatment with dabrafenib/trametinib, at least two available MUGA scans with the first scan at baseline, before or shortly after initiation of treatment with BRAF/MEKi, and the second scan approximately three months after initiation of therapy. Subsequent MUGA scans were routinely performed at least every third month during treatment.
Patients without a baseline MUGA, e.g., due to an urgent need for therapy or recent echocardiography, and patients with no or only one MUGA scan, for example if they did not reach first evaluation due to disease progression, were excluded.
Assessment of cardiac function
Cardiac function was monitored with MUGA scans, that have a low interobserver variability compared to echocardiography, and is a commonly used method to measure LVEF. MUGA scans were carried out as equilibrium radionuclide angiographies (ERNA) performed on a dedicated cardiac cadmium zinc telluride (CZT) SPECT gamma camera, GE Discovery 530c (GE Healthcare, Milwaukee, WI). The CZT dedicated cardiac SPECT camera has a very high reproducibility of LVEF with a low interobserver variation (coefficient of variance 1·7%)[Citation24]. 99mTc-labeled human serum albumin (HSA) was administered intravenously to each patient. An acquisition protocol for multigated acquisition, using 16 bins per R-R interval, set to acquire 600 accepted beats, and a 20% energy window centered on 140 keV was carried out. For image analyses, Xeleris 3 Imaging workstation reorientation software (GE Healthcare, Milwaukee, WI, version no. 3.0562) and Cedars-Sinai QBS processing software (Cedars-Sinai, Los Angeles, CA, revision 2009.0) was used. Each acquisition was processed by two technicians independently of each other and revised by a senior medical doctor if the results for LVEF differed by more than 2 percentage point (pp) [Citation25,Citation26]. The following variables were recorded: left ventricular end-systolic volume (LVESV) and end-diastolic volumes (LVEDV), left ventricular ejection fraction (LVEF), left ventricular peak emptying rate (LVPER) and peak filling rate (LVPFR). Data on height, weight, heart rate (HR), and blood pressure were registered with each scan.
Cardiotoxicity
Cardiotoxicity was defined as a decline in LVEF to <50% or a reduction in LVEF of ≥15 pp. A decline of ≥10 pp to an EF <50% was considered major cardiotoxicity, and a decline in EF of ≥15 pp but remaining >50% was considered minor cardiotoxicity [Citation27]. Patients with an LVEF <50% at baseline were analyzed separately. Cases with cardiotoxicity were further analyzed according to reversibility. Full reversibility was defined as restoration to an LVEF value within 5 pp of baseline, partial reversibility if LVEF improved by at least 10 pp but remained >5 pp below baseline, and no reversibility if LVEF improvement was <10 pp and remained >5 pp below the baseline [Citation28,Citation29].
Other variables
A team of two oncologists, a nuclear radiologist, and a cardiologist selected and agreed upon the studied clinical variables. Patient-, disease-, and treatment-related variables with a potential influence on response to BRAF/MEKi were extracted from DAMMED. Patient-related variables known to be associated with cardiovascular disease (hypertension, hypercholesterolemia, heart failure, atrial fibrillation, diabetes, chronic obstructive lung disease, hyperparathyroidism, hypo- or hyperthyroidism, previous acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, pacemaker, smoking (previous or active), other cancers, previous treatment with chemotherapy or radiation to the truncus) were identified in patient records [Citation21]. Comorbidities were included if the patient received medical treatment for the condition at the time of BRAF/MEKi therapy.
Electrocardiograms at baseline were also analyzed and values for heart rate (HR) (normal value 50–100 beats/min), PR interval (normal value 120–220 ms), QRS duration (normal value <120 ms), and QTcB (normal value <460 ms for men, 480 ms for women) were registered. The rhythm of ECG was classified as sinus rhythm or not. In case of prolonged QRS, the registered QTc (Bazett) was adjusted for the abnormal QRS duration.
Statistical analysis
Continuous variables were expressed as means ± standard deviation or medians according to distribution, and categorical variables were expressed as numbers and percentages. Numerical values were used for LVPER and LVPERadj. Group comparisons on baseline characteristics were performed with the Pearson Chi-squared test for categorical variables and with analysis of variance (ANOVA) adjusted for unequal variances when appropriate for continuous variables. Univariate survival analyses regarding cardiotoxicity, OS, and PFS were carried out on discontinuous variables with the Log-Rank test. A backward stepwise conditional Cox proportional hazards regression analysis on selected variables was performed (Entry p = 0.05; Removal p = 0.01) for the assessment of relative risk (RR) for developing major cardiotoxicity and overall survival. A p-value <0.05 was considered significant. A ROC analysis was performed for variables where assessment of predictive capability regarding development of cardiotoxicity was required.
Kaplan–Meier plots were performed using GraphPad Prism version 5, all other statistical analyses were performed using IBM SPSS Statistics version 25.0.0.2.
Results
Study population
We identified 173 patients with metastatic melanoma treated with dabrafenib/trametinib. Of these, 139 patients (80 men; mean age 60.7 ± 13.5 years) were included in the study as they had at least two MUGA scans during treatment, one of which was done at baseline. Baseline characteristics are summarized in . A total of 700 MUGA scans were performed on included patients; 139 were baseline scans, 139 were first evaluation scans performed approximately three months after initiation of BRAF/MEKi therapy, and 422 scans were performed as scans for second or later evaluation. The mean number of MUGA scans per patient was 5.
Table 1. Baseline characteristics.
Risk of cardiotoxicity
A total of 46 patients (33%) met our criteria for cardiotoxicity: 31 patients (22%) experienced minor cardiotoxicity and 15 patients (11%) experienced major cardiotoxicity (). The decline in LVEF was caused by an increase in LVEDV and LVESV for both minor and major cardiotoxicity. Patients with an LVEF <50% at baseline were excluded from this analysis.
Table 2. Changes in paraclinical values.
Pattern of cardiotoxicity
Minor cardiotoxicity
Three out of 31 patients with minor cardiotoxicity had alterations in the MEKi treatment on suspicion of cardiotoxicity, including dose-reduction or treatment pause. None of these had clinical heart failure or initiated heart failure treatment, and all resumed MEKi therapy without further reduction in LVEF. The remaining patients in this group did not have any symptoms associated with cardiotoxicity nor was treatment altered.
Major cardiotoxicity
For 11 patients experiencing major cardiotoxicity, the decline in LVEF was fully or partially reversible. Of these 11 patients, seven had the MEKi paused, two had a dose-reduction, and two had no alterations in treatment; only one patient developed symptoms of heart failure and received heart failure medication, and all resumed MEKi therapy at a reduced dose.
For the remaining four patients with major cardiotoxicity, the decline in LVEF was not reversible after initial pause or dose-reduction, and three of them developed symptoms of heart failure even after treatment was paused. One patient had MEKi permanently discontinued and recovered later, two patients experienced disease progression shortly after and therefore no further monitoring of LVEF was performed, and one patient died due to comorbidities unrelated to BRAF/MEKi treatment before further measurements of LVEF were performed.
Patients with an LVEF <50% at baseline
Seven patients had a baseline LVEF below 50%; none of them received treatment for heart failure at the time of initiation of BRAF/MEKi therapy. Two patients had a further decrease in LVEF that led to discontinuation of MEKi therapy, and one of them initiated treatment for heart failure. Three patients had reduced, but stable LVEF values. All were treated with an initial 50–75% dose reduction. The remaining two patients had an increase in LVEF to above 50% after the first two measurements while on standard dose MEKi.
Time to cardiotoxicity
The median time to decline in LVEF for all patients with cardiotoxicity was 94 days (). Patients with major cardiotoxicity all experienced a decline in LVEF before the evaluation at six months. Of the patients with minor cardiotoxicity, seven patients experienced a decline in LVEF more than six months after treatment initiation. None of these seven patients had any symptoms of heart failure, and no alterations were done to their treatment.
Table 3. Time to cardiotoxicity.
Risk factors
In the univariate analysis of baseline characteristics, high body mass index was significantly associated with the development of minor cardiotoxicity. However, in multivariate analysis, this association was no longer significant (not shown).
Subgroup analysis of patients with major cardiotoxicity found a low baseline LVPER adjusted for HR (LVPERadj) to be a predictor of major cardiotoxicity (). The ROC curve for LVPERadj as a predictor of major cardiotoxicity has an AUC of 0.757, p = 0.001 (Supplementary Figure 1). No patients with baseline LVPERadj higher than 3.08 mL (42 patients) developed major cardiotoxicity.
Table 4. Multivariate analysis of potential risk factors associated with the development of major cardiotoxicity.
No other associations between any patient-, disease-, and treatment-related variables and the occurrence of major cardiotoxicity were seen. Furthermore, if age and gender adjusted values for LVEDV, LVESV and LVEF were applied (Z-scores) the outcome was unaltered (data not shown).
Overall survival (OS) and progression-free survival (PFS)
Median PFS and OS for patients with no decline in LVEF were 7.4 and 11.7 months, respectively, for patients with minor cardiotoxicity 6.5 and 11.7 months, and for patients with major cardiotoxicity 9.6 and 22.2 months. The Log-Rank test showed a significantly prolonged PFS and a tendency toward a longer OS for patients with major cardiotoxicity compared to patients with no decline in LVEF (p = 0.028 and p = 0.050, respectively). PFS and OS were not significantly different for patients with minor cardiotoxicity compared to patients with no decline in LVEF (p = 0.221 and p = 0.224, respectively) ().
Figure 1. Kaplan-Meier estimation of (A) progression free survival and (B) overall survival in patients with no decline in left ventricular ejection fraction (LVEF), patients with minor cardiotoxicity, and patients with major cardiotoxicity.
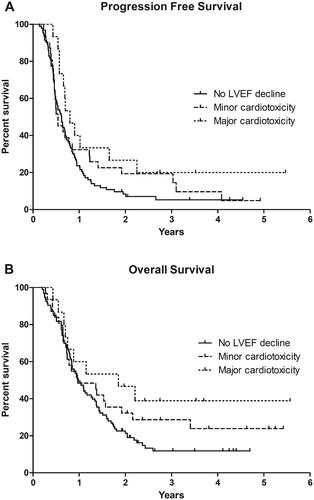
In multivariate analysis of patient characteristic and OS, major cardiotoxicity was not significant (p = 0.051). The analysis showed high diastolic blood pressure (p = 0.012), smoking (p = 0.009), LDH > normal (p = 0.014), and performance status ≥ 2 (p < 0.001) to be associated with short OS (data not shown).
Discussion
In this retrospective analysis of an unselected real-world patient cohort, we found that 33% of melanoma patients treated with BRAFi/MEKi had a decline in LVEF, although only 11% were defined as major cardiotoxicity. The median time to the decline in LVEF was three months, and no patient developed a clinically significant drop in LVEF after six months. Baseline LVPERadj was the only parameter to be associated with the occurrence of major cardiotoxicity in multivariate analysis.
Importantly, the rate of cardiotoxicity in this study is more than three times higher than the approximately 8% reported in clinical trials [Citation4–8,Citation10,Citation13]. We found no association between a decline in LVEF and factors such as age and cardiovascular comorbidity. Instead, this discrepancy might be explained by different definitions of cardiotoxicity and different modalities used to monitor LVEF during treatment. The CZT camera has a wider range of LVEF outcome compared to other methods to assess LVEF [Citation24,Citation30], and this method might include more patients with a decline in LVEF than reported in studies using other modalities to measure LVEF. A recent retrospective analysis on decreased LVEF related to BRAF/MEKi in melanoma defined a decrease in LVEF as a reduction of ≥10 pp to LVEF <55% measured by transthoracic echocardiography and found that 13% of patients treated with combination therapy developed a decline in LVEF [Citation31]. In this study, we found that 11% of patients had a decline of ≥10 pp to an LVEF <50%, which is consistent with the proportion found in other studies [Citation5–8,Citation10,Citation13].
A high HR correlates with a high LVEF [Citation32], and for many of the patients defined as having minor cardiotoxicity, the decline in LVEF occurred simultaneously with a significant decrease in HR (data not shown). A high metabolism, due to a high tumor load, might cause initial high HR [Citation33,Citation34], and the observed decrease in both LVEF and HR is most likely not caused by any cardiotoxic effect, but instead by a reduction in tumor load due to the initial treatment efficacy of BRAF/MEKi. Also, patients experiencing minor cardiotoxicity had a significantly higher LVEF at baseline, which is probably due to the significantly smaller ventricular volumes observed in this patient group, suggesting that these patients had an inferior heart function at baseline. Supporting this, more patients experiencing minor cardiotoxicity had hypertension, hypercholesterolemia, and ischemic heart disease. Therefore, the decline in LVEF for these patients is not necessarily a reflection of cardiotoxicity, since the change in LVEF is affected by a high baseline LVEF due to poor heart function and small ventricular volumes. No patients with minor cardiotoxicity had clinical heart failure or experienced any significant secondary reductions in LVEF regardless of treatment alterations, suggesting that minor cardiotoxicity is not clinically significant and should not lead to dose reduction or discontinuation of BRAF/MEKi treatment but instead continued monitoring of LVEF.
The management of major cardiotoxicity is complex, and both cardiac safety and optimal antineoplastic treatment must be considered. Expert management guidelines suggest pausing MEKi treatment if the patient experiences an absolute decrease in LVEF of 10% or more from baseline and below institutional lower limits of normal [Citation35–37]. If the patient recovers to a normal LVEF, therapy with MEKi can be resumed, and if the patient experiences a continuous decline in LVEF, treatment should be permanently discontinued. In case of heart failure or a decrease in LVEF of 20% or more from baseline and below the lower limit of normal, therapy with MEKi should also be discontinued. However, discontinuing MEKi in favor of cardiac protection should be carefully balanced against the potential antineoplastic benefit. Especially, if the cardiotoxicity is asymptomatic. In our study, only few patients experienced irreversible cardiotoxicity; however, in all cases monitoring of LVEF stopped due to progression of metastatic disease or death from other causes, therefore it remains unknown whether these patients could have recovered later. In addition to this, major cardiotoxicity was reversible for several patients, including two patients that continued therapy on a full dose. Patients managed either with a pause in treatment, or a dose reduction might have recovered even if they had remained on the full dose. Despite that, a decline in LVEF can be severe and individualized decisions and repeated measurements of LVEF for the patients experiencing major cardiotoxicity are required.
Patients with a baseline LVEF <50% were treated initially with a reduced MEKi dose, and therefore it is not possible to conclude on the risk of treating these patients with full-dose therapy. Still, this study shows that with close monitoring of LVEF and symptoms of heart failure, a baseline LVEF <50% is manageable and should not be a contraindication to therapy.
All patients with major cardiotoxicity experienced the decline in LVEF before the evaluation at six months with a median time from initiation of BRAF/MEKi treatment to the development of a decline in LVEF of 83 days. A toxicological report found a very similar median time to decrease in LVEF of 86 days (range 27–253) in patients who received dabrafenib and trametinib [Citation14]. OS at six months did not differ between the three groups (), indicating that early onset of decreased LVEF is not caused by poor prognosis for patients at risk of developing cardiotoxicity. Therefore, routine monitoring of LVEF for patients treated with BRAF/MEKi could be safely halted after six to nine months of treatment if no signs of heart failure or a decline in LVEF had occurred.
The analysis of potential risk factors for a decline in LVEF showed an association between a low baseline LVPERadj and an increased risk of major cardiotoxicity. To our knowledge, this parameter has not previously been investigated, wherefore no other data are available for comparison. Baseline LVPERadj could potentially be used to identify patients with low values as having an increased risk of developing major cardiotoxicity and patients with higher values as having low risk. Potentially, a low LVPERadj expresses a myocardium with preexisting low contractility, which leaves it more vulnerable to the cardiotoxic effects of BRAF/MEKi, in particular those associated with cardiomyopathy and hypotension [Citation15,Citation16,Citation18]. The analysis showed no other associations between any patient-, disease-, or treatment-related variables and the occurrence of major cardiotoxicity.
Interestingly, we found that the patients who developed major cardiotoxicity had a significantly improved PFS and a tendency toward an improved OS. All cases of major cardiotoxicity occurred within the first six months of treatment, and these patients did not receive a higher cumulative dose within this time period than patients with no decline in LVEF, indicating that neither the occurrence of major cardiotoxicity nor the longer PFS and OS for this group is explained by a cumulatively higher dose or a longer time of exposure to BRAF/MEKi. However, only relatively few patients in this study experienced major cardiotoxicity, and the difference in outcome might be due to sample size; also, another study found no difference in OS for patients with or without a decline in LVEF [Citation31]. Poor prognostic factors such as a high performance status and a high LDH, were, as expected, also associated with a decrease in PFS and OS.
The retrospective design is a limitation of this study wherefore findings, such as the LVPERadj and the association to BRAF/MEKi induced cardiotoxicity, must be evaluated in prospective trials. Also, our relatively small patient population with only 15 patients diagnosed with major cardiotoxicity must be considered when evaluating data. The fact that preexisting cardiac disease was not associated with the occurrence of major cardiotoxicity calls for attention and results regarding the association of outcome and cardiotoxicity must be interpreted with caution.
In conclusion, the median time to the development of major cardiotoxicity was approximately three months, and no patients experienced a clinically significant decline in LVEF after six months of treatment. Therefore, we suggest that monitoring LVEF during treatment with BRAFi/MEKi is only necessary during the first six to nine months of therapy unless a drop in LVEF has been seen before that time. No associations between any baseline characteristics and the development of either minor or major cardiotoxicity were seen, except for baseline HR-adjusted LVPER.
Supplemental Material
Download MS Word (25.1 KB)Disclosure statement
EE received honoraria from BMS, Pierre Fabre, and Novartis for lectures and consultancies, and from MSD for travel/conference expenses. IMS has an advisory board relationship with or lectured for Roche, Novartis, MSD, Celgene, Incyte, TILT bio, Pfizer, BMS and AstraZeneca and has received limited grants for translational research from BMS, Roche and Novartis. The other authors declare no conflicts of interest.
Additional information
Funding
References
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984.
- Mandalà M, Voit C. Targeting BRAF in melanoma: biological and clinical challenges. Crit Rev Oncol Hematol. 2013;87(3):239–255.
- Spagnolo F, Ghiorzo P, Orgiano L, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther. 2015;8:157–168.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703.
- Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–1639.
- Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–1260.
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2017;28(suppl_4):iv119–iv142.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365.
- Livingstone E, Zimmer L, Vaubel J, et al. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chinese Clin Oncol. 2014;3(3):29.
- Mincu RI, Mahabadi AA, Michel L, et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):1–13.
- Shah RR, Morganroth J. Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Safety. 2015;38(8):693–710.
- Bronte E, Bronte G, Novo G, et al. Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Pharmacol Ther. 2018;192:65–73.
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546.
- Rossello X, Yellon DM. The RISK pathway and beyond. Basic Res Cardiol. 2018;113(1):1–5.
- Bronte E, Bronte G, Novo G, et al. What links BRAF to the heart function? New insights from the cardiotoxicity of BRAF inhibitors in cancer treatment. Oncotarget. 2015;6(34):35589–35601.
- Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91(9):776–781.
- Abdel-Rahman O, ElHalawani H, Ahmed H. Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: a comparative systematic review and meta-analysis. J Glob Oncol. 2015;1(2):73–82.
- Chen-Scarabelli C, McRee C, Leesar MA, et al. Comprehensive review on cardio-oncology: role of multimodality imaging. J Nucl Cardiol. 2017;24(3):906–935.
- Guha A, Jain P, Fradley MG, et al. Cardiovascular adverse events associated with BRAF versus BRAF/MEK inhibitor: cross-sectional and longitudinal analysis using two large national registries. Cancer Med. 2021;10(12):3862–3872.
- Ellebaek E, Svane IM, Schmidt H, et al. The Danish metastatic melanoma database (DAMMED): a nation-wide platform for quality assurance and research in real-world data on medical therapy in Danish melanoma patients. Cancer Epidemiol. 2021;73: 101943.
- Jensen MM, Haase C, Zerahn B. Interstudy repeatability of left and right ventricular volume estimations by serial-gated tomographic radionuclide angiographies using a cadmium-zinc-telluride detector gamma camera. Clin Physiol Funct Imaging. 2015;35(6):418–424.
- Rydberg J, Andersen J, Haarmark C, et al. The influence of anthropometric and basic circulatory variables on count rate in cadmium-zinc-telluride SPECT gated radionuclide angiography. J Nucl Cardiol. 2019;26(6):1974–1980.
- Hansen NL, Haarmark C, Zerahn B. Ventricular peak emptying and filling rates measured by gated tomographic radionuclide angiography using a cadmium–zinc–telluride SPECT camera in chemotherapy-naïve cancer patients. J Nucl Cardiol. 2020;27(4):1193–1201.
- Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer addressing the unresolved issues. Circulation. 2012;126(23):2749–2763.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–939.
- Perez IE, Alam ST, Hernandez GA, et al. Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol. 2019;13:117954681986644.
- Haarmark C, Haase C, Jensen MM, et al. Pre-chemotherapy values for left and right ventricular volumes and ejection fraction by gated tomographic radionuclide angiography using a cadmium-zinc-telluride detector gamma camera. J Nucl Cardiol. 2016;23(1):87–97.
- Berger M, Amini-Adlé M, Maucort-Boulch D, et al. Left ventricular ejection fraction decrease related to BRAF and/or MEK inhibitors in metastatic melanoma patients: a retrospective analysis. Cancer Med. 2020;9(8):2611–2620.
- Erbel R, Schweizer P, Krebs W, et al. Effects of heart rate changes on left ventricular volume and ejection fraction: a 2-dimensional echocardiographic study. Am J Cardiol. 1984;53(4):590–597.
- Von Haehling S, Lainscak M, Kung T, et al. Non-invasive assessment of cardiac hemodynamics in patients with advanced cancer and with chronic heart failure: a pilot feasibility study. Arch Med Sci. 2013;9(2):261–267.
- Anker MS, Ebner N, Hildebrandt B, et al. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study. Eur J Heart Fail. 2016;18(12):1524–1534.
- Knispel S, Zimmer L, Kanaki T, et al. The safety and efficacy of dabrafenib and trametinib for the treatment of melanoma. Expert Opin Drug Saf. 2018;17(1):73–87.
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7(2):122–136.
- Totzeck M, Schuler M, Stuschke M, et al. Cardio-oncology – strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163–175.
