Introduction
Even though the incidence of squamous cell carcinoma of the anus (SCCA) is rising [Citation1], it is still considered a rare malignancy representing less than 3% of all gastrointestinal cancers [Citation2]. The majority of patients diagnosed with SCCA presents with localised or locally advanced disease, and the standard treatment, consisting of chemo-radiotherapy (CRT), is well established [Citation3,Citation4]. Palliative chemotherapy is the standard treatment option for advanced SCCA (inoperable locoregional recurrence or metastatic SCCA (mSCCA)), but the evidence is limited with only one published randomised trial on chemotherapy in the advanced setting (InterAAct) [Citation5]. This study has established carboplatin and paclitaxel as first-line chemotherapy choices for advanced SCCA.
Synchronous mSCCA is only seen in less than 10% of primary diagnosed SCCA [Citation6], and the most frequent sites of distant metastases are liver, lung, and lymph nodes outside the pelvis [Citation7]. Definitive treatment of oligometastatic disease is a well-established option for other diagnoses with the possibility of long-term disease control [Citation8,Citation9]. The literature on definitive therapy for synchronous mSCCA is however extremely sparse and is characterised by limited case reports [Citation10–15] and a small number of mainly retrospective studies with small sample sizes often comprising both synchronous and metachronous metastases [Citation16–22]. Consequently, there is an unmet need for evidence to guide clinicians in the treatment of patients diagnosed with synchronous mSCCA.
The different approaches used in the literature, on definitive management of synchronous mSCCA, are either metastases-directed therapy (metastasectomy, radiofrequency ablation (RFA) or stereotactic body radiation therapy (SBRT)) in combination with CRT or, in cases of lymph node metastases outside the pelvis, CRT with extended radiotherapy fields. To our knowledge, no literature exists on induction chemotherapy (ICT) as a treatment option in the management of definitive treatment for synchronous mSCCA.
The purpose of this study was to evaluate the outcome of patients diagnosed with synchronous mSCCA treated with definitive therapy comprising ICT in combination with definitive (chemo)radiotherapy and potential metastases-directed therapy and to review the literature on definitive treatment for synchronous mSCCA.
Methods
Patients
Patients with a histopathological diagnosis of primary SCCA with synchronous distant metastases, treated with ICT as part of a definitive treatment strategy between 2000 and 2018 were included in this study. Patients were identified from treatment registers comprising patients treated with ICT at one of the three Danish Centres (Department of Oncology, Aarhus University Hospital; Department of Oncology, Vejle Hospital, University Hospital of Southern Denmark; or Department of Oncology, Herlev and Gentofte Hospital). A retrospective data collection from medical records was performed. Information on pre-treatment patient- and tumour characteristics, treatment, response to treatment, and outcome data were collected. Approval for data collection was given by the Danish Patient Safety Authority (3-3013-2447/1) and the Danish Data Protection Agency (1-16-02-66-18).
The diagnostic workup consisted of a clinical examination and imaging (depending on the centre and time period chest x-ray, computer tomography (CT), magnetic resonance imaging (MRI), and/or 18-fluorodeoxyglucose positron emission tomography (FDG-PET) were used).
Diagnosis of distant metastases was based on imaging and/or biopsy. Liver metastases were considered diagnostic on CT. Skin metastases were defined as elements with no connection to the primary tumour and biopsied independently. Local metastases-directed therapy was performed based on multidisciplinary team (MDT) conference decisions.
Treatment
Patients were treated with cisplatin and 5-flourouracil based ICT prior to (chemo)radiotherapy. Two regimens were used: either cisplatin (37.5 mg/m2 intravenously (iv.), ifosfamide (2.0 mg/m2 iv.), and 5-flourouracil (500 mg/m2 iv.) day one and two in combination with leucovorin (60 mg/m2 iv.) (CILF) or cisplatin (60 mg/m2 iv.) administered at day one and 5-floururacil (1000 mg/m2) administered with 24 h continuous infusion days 1–4 (cis-5-FU). Supportive therapy to patients receiving ifosfamide included mesna (500 mg/m2 iv. administered before ifosfamide and 1 g/m2 orally at two and six hours after ifosfamide). Hydration and antiemetic were administered by local protocol. Granulocyte colony-stimulating factor (G-CSF) was not used routinely but administered at the discretion of the physician.
Patients who received metastases-directed therapy were treated with surgery (liver or lung resection), RFA and/or SBRT of the liver.
Patients were treated in supine position with radiotherapy delivered with either 3 D conformal (2000–2007) or intensity-modulated radiation therapy (IMRT) (2007–2018). Prescribed doses to tumour and pathological lymph nodes were 50.4–64 Grey (Gy) in 28–32 fractions five fractions per week and prescribed doses to the elective lymph node area were 49.5–51.2 Gy in 28–32 fractions. Metastases treated within the radiation fields were included in the high-dose area. Concomitant chemotherapy was either cisplatin- or fluoropyrimidine-based or a combination of these.
Literature search
A literature search in PubMed was performed. Relevant literature in English on mSCCA treated with a definitive treatment strategy was identified using different combinations of the following search- and/or mesh words: anal cancer, squamous cell carcinoma, squamous cell carcinoma of the anus, multimodality treatment, neoplasm metastases, liver metastases and/or radiofrequency ablation. The reference list of relevant publications was reviewed, and potentially relevant publications were identified.
Statistical analysis
Patients were divided into two groups comprising patients with organ metastases (liver, lung, bone, and skin) or patients with distant lymph node metastases (retroperitoneal lymph nodes or para aortic-/common iliac lymph nodes). Patient- and tumour characteristics within the two groups were presented with descriptive statistics as numbers (n) and percentage (%) or medians with ranges, and the two groups were compared using Wilcoxon Mann-Whitney test for categorical variables, Fishers exact for binary variables and two-sample t-test when comparing two means. A p-value of 0.05 was considered statistically significant.
The Kaplan-Meier method was used to calculate and present survival functions. Overall survival (OS) was calculated from the date of diagnoses to the date of death or the date of last observation. Disease-free survival (DFS) was calculated from the date of diagnoses to the date of progression, recurrence (locoregional or distant), date of death or date of last observation whichever came first. For patients who completed definitive treatment (n = 16) time to first recurrence and time to first distant failure were calculated from date of last radiotherapy dose to date of first recurrence or date of first distant failure, respectively. Survival is presented in percentage and as median with 95% confidence intervals (CI). Survival functions within the two groups (organ metastases versus distant lymph node metastases) were compared using a log-rank test. All statistical analyses were performed using STATA 16.1 (STATA/IC16.1, Stata Corp LP, Texas, College Station, USA).
Results
Pre-treatment characteristics
From the three National Centres 19 patients (Aarhus University Hospital (n = 5), Vejle Hospital (n = 4) and Herlev Hospital (n = 10)) met the inclusion criteria of synchronous mSCCA treated with ICT prior to definitive therapy. In , pre-treatment characteristics are presented. The locations of distant metastases were liver (n = 5), lung (n = 1), bone (n = 1), skin (n = 3), retroperitoneal lymph nodes (n = 4), and para aortic-/common iliac lymph nodes (n = 5). In seven cases biopsy of the distant metastases was performed (liver n = 2 (in two cases of liver metastases information on biopsy was not available), lung n = 1, skin n = 3, retroperitoneal lymph node n = 1). The bone metastasis was located to the sacrum with no connection to the primary tumour and was diagnosed on all imaging modalities (CT, MRI, and PET-CT). Biopsy was not performed, but the lesion was classified as malignant based on the diagnostic imaging, response to PET-CT uptake, and change from lytic to sclerotic on imaging.
Table 1. Pre-treatment characteristics.
Treatment
All patients received ICT with either CILF (n = 18) or with cis-5-FU (n = 1). Median number of cycles was 3 (range 2–9). Four patients with either lung or liver metastases had the local metastases-directed treatment performed (surgery, RFA, and/or SBRT) before ICT (n = 1) or before/during radiotherapy (n = 3). In 13 cases, distant metastases were included in the irradiated fields. In two cases, information on potentially local metastases-directed therapy was not available.
Radiotherapy treatment is summarised in Supplementary Table S1. Patients received either chemo-radiotherapy (n = 9) or radiotherapy alone (n = 10) with either 3D conformal (n = 8) or IMRT (n = 11). Except for liver and lung metastases, distant metastases were included in the irradiated area.
Treatment response, recurrence and outcome
Clinical response evaluation after completing ICT was available in 16 patients. Of these, five had a complete local tumour response, and nine had a partial local tumour response. In two patients, a complete pathological response of lever metastases was observed, the remaining cases were clinically/radiologically evaluated.
After completion of definitive treatment, 16 patients achieved complete response including one patient who underwent salvage surgery for persistent disease. The remaining three patients received palliative treatment. An overview of treatment and response is depicted in Supplementary Figure S1.
Figure 1. Kaplan-Meier survival curves. (A) Overall survival, (B) Overall survival according to organ metastases (liver, lung, bone, and skin) versus distant lymph node metastases (retroperitoneal lymph nodes or para aortic-/common iliac lymph nodes), (C) Disease-free survival, and (D) Disease-free survival according to organ metastases versus distant lymph node metastases. LN: lymph node.
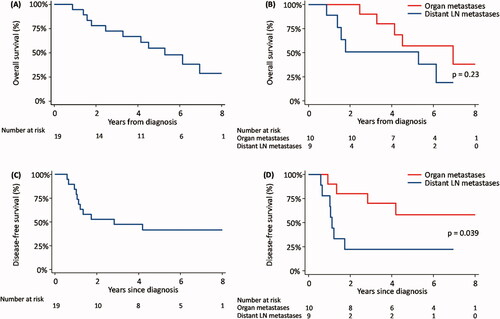
The median follow-up time was 4.5 years (range 0.9–17). Three patients did not achieve complete response after completing definitive (chemo)radiotherapy. During follow-up, eight patients experienced recurrence with seven patients diagnosed with distant failure. Five patients had locoregional recurrence including one patient with locoregional failure only (Supplementary Table S2). For patients who had a complete response after definitive treatment (n = 16) the median time to the first recurrence was 10 months (range 6–29) and the median time to first distant failure was 24 months (range 6–57).
Table 2. Overview of the literature on a definitive treatment strategy for metastatic SCCA.
Survival functions are illustrated in . Looking at the entire group the 3- and 5-year OS was 72% and 55%, respectively, and 3- and 5-year DFS was 47% and 41%, respectively. The median OS was 4.5 years (95%CI: 2.3–6.1) and the median PFS was 2.8 years (95%CI: 1.1–5.6). When dividing patients into two groups with either organ metastases (liver, lung, skin or bone) or distant lymph node metastases (para aortic-, common iliac-, or retroperitoneal lymph nodes) a significant better DFS in patients with organ metastases compared to distant lymph node metastases was seen, p = 0.039. No significant difference in OS was seen between the two groups (p = 0.23).
Literature review
An overview of the literature on definitive treatment for mSCCA is presented in . Eight studies on mSCCA treated with metastases-directed therapy were identified. Of these studies, five included patients with different metastatic sites treated with organ-directed therapies [Citation16–20]. All of these studies were retrospective, and have all included both synchronous and metachronous mSCCA. Three studies investigating extended radiotherapy fields for patients with para-aortic lymph nodes were identified [Citation21–23]. Of these, one was prospective with 30 patients and the two others were retrospective studies; one with six patients and the other with four patients, all with synchronous distant lymph node metastases treated with extended radiotherapy fields. In addition, six case reports of patients with mSCCA were identified [Citation10–15].
The use of ICT was difficult to assess. Some of the studies reporting on metastases-directed therapies reported the use of chemotherapy prior to metastases-directed therapy, but the use in the synchronous setting alone was not possible to assess from the publications. In the studies with extended radiotherapy fields, no ICT was used, however, information on ICT for the four patients in Nilsson et al. [Citation23] was not available.
Discussion
This retrospective study implies that ICT in combination with definitive (chemo)radiotherapy and potentially local metastases-directed therapy is feasible and a possible definitive treatment option in selected patients with synchronous mSCCA. We found a 3- and 5-year OS of 72% and 55%, respectively with a median OS of 4.5 years. These results suggest that it is possible to obtain long-term survival in selected patients with synchronous mSCCA. In comparison, 5-year OS of patients with advanced SCCA receiving standard palliative chemotherapy has been reported to approximately 30% calculated from the date of diagnoses and 15% from the date of first-line chemotherapy [Citation24]. Recently new palliative regimens have been investigated in prospective trials where an increase in 5-year OS has been reported [Citation5,Citation25]. In the InterAAct study, patients were randomised to either carboplatin/paclitaxel or cisplatin/5-FU with a median OS in the carboplatin/paclitaxel group of 20 months and 12.3 months in the cisplatin/5-FU group [Citation5]. In addition, the combination of docetaxel, cisplatin, and 5-FU (standard or modified regimen) have been published in a phase II study (HPV01 and HPV02) as an option for first-line chemotherapy with a median OS of 39.2 month in the pooled analysis of both Epitopes-HPV01 and -02 [Citation26].
In the ESMO-ESSO-ESTRO Anal Cancer Guideline from 2014 [Citation27], it is recommended that patients with advanced SCCA are discussed at an MDT conference to explore possible treatment options. A multidisciplinary approach was investigated in a retrospective study comparing patients with mSCCA receiving standard palliative chemotherapy to patients treated with a multidisciplinary approach. Median OS was 17 months for patients receiving standard palliative chemotherapy versus a median OS of 4.4 years in the group of patients receiving a multidisciplinary approach [Citation19].
When treating patients with synchronous mSCCA different approaches in the ‘oncological toolbox’ are available depending on the metastatic site, but very few studies exist to support treatment decisions. A literature search in PubMed revealed five publications on organ-directed therapy as part of a definitive treatment strategy for mSCCA [Citation16–20]. Firstly, all publications were retrospective, and in contrast to our dataset, all five publications describe a pooled analysis of patients with both synchronous and metachronous distant metastases, rendering a direct comparison with our data difficult. Two publications on extended radiotherapy fields in cases of synchronous distant lymph node metastases were identified one being a retrospective study and one a prospective study [Citation21,Citation22]. In addition, in a publication on patterns of failure in a retrospective cohort, four patients with synchronous distant lymph node metastases were included and treated with extended fields [Citation23]. None of these study patients received ICT.
Due to the retrospective and heterogeneous nature of the literature, a direct comparison of outcome data between the studies must be done with caution. We present an overall survival of 54 months, comparable to the range of 22–53 months in available datasets [Citation16–19] and a 3-year OS rate of 72% compared to 63% and 67% [Citation21,Citation22]. Altogether, these results are encouraging in metastatic settings. However, the study cohorts, clinical settings and treatment approaches vary and cannot consequently be directly compared.
We observed a better DSF in patients with organ metastases compared to patients with distant lymph node metastases, but no significant difference in OS. In contrast to our findings, organ metastases in stage IV cervical cancer treated with both definitive and palliative intent were associated with a worse OS compared to distant lymph node metastases [Citation28]. Haematogenous and lymphatic dissemination is part of the hallmark of cancer [Citation29] and many factors, such as genetic and microenvironmental factors, are responsible for the metastatic potential of tumours [Citation30]. The observed difference in DFS in our cohort is an interesting biological observation, but any conclusions on prognosis in relation to haematogenous versus lymphatic metastatic spread cannot be drawn based on this study.
Patients in our cohort of real-world data have been selected for a definitive treatment strategy by the treating clinicians, and our data raises several questions to address in future studies. First, is there a biological difference between patients with advanced lymph node status and those with limited distant metastatic spread, indicating that patients with multiple lymph nodes are less likely to be candidates for a curative approach? Second, can we identify pre-treatment features such as human papilloma virus status and other molecular markers with prognostic information? In brief, we need more knowledge on patient selection and international collaboration is warranted in this rare setting. Clinical data and biomarker studies are needed to define criteria for candidates who will benefit from an intensified definitive treatment strategy.
Another interesting aspect for future studies in this specific setting is the investigation of the abscopal effect of synchronous distant metastases. Our study was not designed to address this biological phenomenon, but future prospective studies are warranted.
This study is the first to report on a cohort of only synchronous mSCCA with both organ metastases and distant lymph node metastases. Limitations are its retrospective nature and the small sample size. Nevertheless, this study represents real-world nationwide clinical data on a definitive treatment option, including the use of ICT and potentially metastases-directed therapy prior to definitive (chemo)radiotherapy. Our data support an MDT-based active clinical approach in the management of synchronous mSCCA.
With this study, we present proof of principle for a definitive treatment strategy in selected patients with synchronous mSCCA. New chemotherapy regimens in the advanced setting call for studies investigating the optimal ICT regimen for these patients. The literature on a definitive treatment strategy for mSCCA is very limited, but the results are promising and should be further investigated in prospective, and preferable, international studies.
Supplemental Material
Download MS Word (19.3 KB)Supplemental Material
Download MS Word (18.8 KB)Supplemental Material
Download JPEG Image (42.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Islami F, Ferlay J, Lortet-Tieulent J, et al. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46(3):924–938.
- Nelson VM, Benson AB. Epidemiology of anal canal cancer. Surg Oncol Clin N Am. 2017;26(1):9–15.
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for research and treatment of cancer radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15(5):2040–2049.
- UKCCCR Anal Cancer Working Party. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348:1049–1054.
- Rao S, Sclafani F, Eng C, et al. International rare cancers initiative multicenter randomized phase II trial of cisplatin and fluorouracil versus carboplatin and paclitaxel in advanced anal cancer: InterAAct. J Clin Oncol. 2020;38(22):2510–2518.
- Guren MG, Aagnes B, Nygård M, et al. Rising incidence and improved survival of anal squamous cell carcinoma in Norway, 1987-2016. Clin Colorectal Cancer. 2019;18(1):e96–103–e103.
- Cummings BJ. Metastatic anal cancer: the search for cure. Onkologie. 2006;29(1-2):5–6.
- Gutiontov S, Pitroda S, Weichselbaum R. The spectrum of metastasis: an opportunity for cure? Semin Radiat Oncol. 2021;31(3):174–179.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Boldrini L, Cellini F, Manfrida S, et al. Use of indirect target gating in magnetic resonance-guided liver stereotactic body radiotherapy: case report of an oligometastatic patient. Cureus. 2018;10(3):e2292.
- Sousa TT, Santos B do N, Belotto M, et al. Successful hepatectomy for metastatic squamous cell carcinoma of the anal canal-a case report. J Gastrointest Oncol. 2016;7(6):E103–6.
- Sakanaka K, Ishida Y, Mizowaki T. A case report of locally advanced anal cancer with solitary cutaneous nodular metastasis in the ipsilateral labia majora treated with definitive chemoradiotherapy. Case Rep Oncol. 2019;12(3):721–727.
- Kim SS, Kim GE, Ko AH. A patient with HIV-Associated metastatic anal squamous cell carcinoma receiving multimodality therapy with curative intent: case report and review of the literature. J Gastrointest Cancer. 2017;48(1):94–99.
- Tokar M, Bobilev D, Zalmanov S, et al. Combined multimodal approach to the treatment of metastatic anal carcinoma: report of a case and review of the literature. Onkologie. 2006;29(1-2):30–32.
- Dall'Armellina F, Perret A, Smolenschi C, et al. Hepatic arterial infusion of chemotherapy as an option in a multimodal treatment of metastatic squamous cell carcinoma of the anus. Eur J Cancer. 2021;142:147–149.
- Goldner M, Platoff R, Betances A, et al. Role of metastasectomy for liver metastasis in stage IV anal cancer. Am J Surg. 2021;221(4):832–838.
- Sclafani F, Hesselberg G, Thompson SR, et al. Multimodality treatment of oligometastatic anal squamous cell carcinoma: a case series and literature review. J Surg Oncol. 2019;119(4):489–496.
- Evesque L, Benezery K, Follana P, et al. Multimodal therapy of squamous cell carcinoma of the anus with distant metastasis: a single-institution experience. Dis Colon Rectum. 2017;60(8):785–791.
- Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget. 2014;5(22):11133–11142.
- Pawlik TM, Gleisner AL, Bauer TW, et al. Liver-directed surgery for metastatic squamous cell carcinoma to the liver: results of a multi-center analysis. Ann Surg Oncol. 2007;14(10):2807–2816.
- Hodges JC, Das P, Eng C, et al. Intensity-modulated radiation therapy for the treatment of squamous cell anal cancer with Para-aortic nodal involvement. Int J Radiat Oncol Biol Phys. 2009;75(3):791–794.
- Holliday EB, Lester SC, Harmsen WS, et al. Extended-field chemoradiation therapy for definitive treatment of anal canal squamous cell carcinoma involving the Para-Aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2018;102(1):102–108.
- Nilsson MP, Nilsson ED, Johnsson A, et al. Patterns of recurrence in anal cancer: a detailed analysis. Radiat Oncol. 2020;15(1):1–10.
- Sclafani F, Morano F, Cunningham D, et al. Platinum-fluoropyrimidine and paclitaxel-based chemotherapy in the treatment of advanced anal cancer patients. Oncologist. 2017;22(4):402–408.
- Kim S, François E, André T, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19(8):1094–1106.
- Kim S, Meurisse A, Spehner L, et al. Pooled analysis of 115 patients from updated data of Epitopes-HPV01 and Epitopes-HPV02 studies in first-line advanced anal squamous cell carcinoma. Ther Adv Med Oncol. 2020;12:1–11.
- Glynne-Jones R, Nilsson PJ, Aschele C, ESTRO, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;111(3):330–339.
- Oishi S, Kudaka W, Toita T, et al. Prognostic factors and treatment outcome for patients with stage IVB cervical cancer. Anticancer Res. 2016;36(7):3471–3475.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
- Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr). 2016;39(5):397–410.
