Abstract
Background
Presence of comorbid diseases at time of cancer diagnosis may affect prognosis. We evaluated the impact of comorbidity on survival of patients diagnosed with renal cell carcinoma (RCC), overall and among younger (<70 years) and older (≥70 years) patients.
Methods
We established a nationwide register-based cohort of 7894 patients aged ≥18 years diagnosed with RCC in Denmark between 2006 and 2017. We computed 1- and 5-year overall survival and hazard ratios (HRs) for death according to the Charlson Comorbidity Index (CCI) score.
Results
Survival decreased with increasing CCI score despite an overall increase in survival over time. The 5-year survival rate of patients with no comorbidity increased from 57% among those diagnosed in 2006–2008 to 69% among those diagnosed in 2012–2014. During the same periods, the survival rate increased from 46% to 62% among patients with a CCI score of 1–2 and from 39% to 44% for those with a CCI score of ≥3. Patients with CCI scores of 1–2 and ≥3 had higher mortality rates than patients with no registered comorbidity (HR 1.15, 95% CI 1.06–1.24 and HR 1.56, 95% CI 1.40–1.73). Patterns were similar for older and younger patients. Particularly, diagnoses of liver disease (HR 2.09, 95% CI 1.53–2.84 and HR 4.01, 95% CI 2.44–6.56) and dementia (HR 2.16, 95% CI 1.34–3.48) increased mortality.
Conclusion
Comorbidity decreased the survival of patients with RCC, irrespective of age, despite an overall increasing survival over time. These results highlight the importance of focusing on comorbidity in this group of patients.
Background
The incidence of renal cell carcinoma (RCC) is increasing worldwide. This is due partly to an increasing number of radiological investigations for other diseases, which leads to incidental detection of small renal tumors [Citation1]. Most cases of RCC are localized; however, up to 20–30% of the patients will develop metastatic disease after surgery [Citation2]. The 5-year relative survival rate of patients with RCC is 75% [Citation3]. Worldwide, more than 140 000 renal cancer-related deaths are reported annually [Citation4].
Comorbidity has been found to be associated with a longer hospital stay, complications [Citation5,Citation6], and mortality [Citation7–15] after renal cancer surgery. Most previous studies were relatively small, and none of them focused on comorbidity as a prognostic factor in a nationwide cohort of patients with RCC with long-term follow-up. A previous Danish register-based study found that comorbidity was a negative prognostic factor in a cohort of patients with RCC diagnosed between 1995 and 2006; [Citation8] however, patients from only two of five Danish regions were included. This study found temporal changes in both the prevalence of comorbidity at the time of cancer diagnosis and in survival according to comorbidity status [Citation8]. Such changes may have important consequences for health care planning and treatment, especially for the elderly, in whom polypharmacy and comorbidity are frequent.
It is of importance to follow the development of prevalence and the prognostic effect of comorbidity in a more recent nationwide cohort of patients. It is also relevant to investigate whether the effect of comorbidity on survival varies between older and younger patients. Such knowledge can contribute to the base of knowledge that is needed in treatment decisions for older patients with RCC. Therefore, this nationwide population-based study aimed to examine comorbidity prevalence among patients with RCC diagnosed between 2006 and 2017; and to evaluate the prognostic impact of comorbidity on survival, overall as well as among younger (<70 years) and older (≥70 years) patients.
Material and methods
This register-based cohort study with up to 13 years of follow-up combined data from national registers for the entire study population, as all residents of Denmark are registered with a unique personal identification number, which is used in Danish registers, allowing linkage of data across registers at an individual level [Citation16].
Study population
We established a population of all patients aged ≥18 years diagnosed with primary RCC in Denmark between 2006 and 2017. Eligible patients were those with RCC verified by histology or cytology in the Danish Pathology Register, which contains information on all histological and cytological examinations performed [Citation17]. We used the following codes to identify patients: SNOMED code T71 (kidney) in combination with M80103-M958x3 (all malignant tumors), except M89603 (nephroblastoma) and M81203 (urothelial carcinoma), or the specific code AEF4510 (metastasis or recurrence derived from the kidney). The patients also had to be recorded with a diagnosis of RCC in the Danish National Patient Register (ICD-10 code C64), which holds information on all diagnoses registered at inpatient and outpatient hospital contacts [Citation18]. All patients were followed until emigration, death, or 31 December 2019, whichever came first.
Exposures and outcomes
Information on preexisting diseases up to 10 years before the RCC diagnosis was obtained from the Danish National Patient Register. From these data, we computed the Charlson Comorbidity Index (CCI), which includes 19 medical conditions (listed in Supplementary Table 1). A positive predictive value > 90% has been found for almost all ICD-10 diagnostic codes used to ascertain the CCI in the Danish National Patient Register [Citation19]. Each condition is weighted according to its potential impact on mortality, and the CCI score is calculated as the sum of those weights (from 0 to 6). A higher CCI score indicates increased severity of comorbid conditions [Citation20,Citation21]. In this study, we categorized CCI scores into groups 0, 1–2, and ≥3.
Table 1. Characteristics of 7894 patients diagnosed with renal cell carcinoma in Denmark, 2006–2017 according to their Charlson Comorbidity Index score.
Information on the stage of RCC was obtained from the Danish Cancer Registry and categorized as localized/regional stage and advanced stage [Citation22].
Data on vital status were obtained from the Civil Registration System, in which dates of death and emigration are recorded continuously for all residents [Citation23].
The study population was divided into younger and older patients with 70 years as the cutoff point, as in previous studies of cancer patients [Citation24].
Statistical analysis
The prevalence of CCI scores (0, 1–2, ≥3) at the date of RCC diagnosis was calculated among patients diagnosed during four calendar periods: 2006–2008, 2009–2011, 2012–2014, and 2015–2017. Further, we computed the prevalence of all 19 individual conditions included in the CCI for the total study population.
Kaplan-Meier curves for overall survival were computed according to CCI scores and calendar period of diagnosis. Overall 1- and 5-year survival and 95% confidence intervals were calculated by the Kaplan-Meier product-limit method for the entire study population and patients aged <70 years and ≥70 years according to the period of diagnosis and stage of the disease.
We used Cox proportional hazards regression analysis to compare mortality (hazard of death) in patients with a CCI score of 0 with that of patients with a score of 1–2 or ≥3, respectively. We calculated 1- and 5-year HRs as well as HRs for the entire follow-up period. In a second analysis, we compared mortality among renal cell carcinoma patients with and without each of the 19 conditions included in the CCI with and without mutual adjustment of all the conditions. All analyses were stratified by sex and age at diagnosis (5-year age groups). Overall analyses were performed for the entire population, and separate analyses were conducted for patients <70 years and those ≥70 years. The proportional hazards assumptions were evaluated by assessing log-minus-log survivor curves.
Analyses were conducted with SAS®, version 9.4.
Results
We included 7894 patients with RCC diagnosed in Denmark between 2006 and 2017. Of these, 5179 (66%) were men, and 2715 (34%) were women. The median age at diagnosis was 66 years (IQR: 15), and 36% of the patients were ≥70 years. The number of patients with RCC diagnosed per year increased during the study period, from 1402 in 2006–2008 to 2528 in 2015–2017 ().
Table 2. Overall 1- and 5-year survival rates and 95% confidence intervals (CIs) for 7894 patients diagnosed with renal cell carcinoma in Denmark in 2006–2017 by Charlson Comorbidity Index score and calendar period of diagnosis.
In all, 2879 (36%) of the patients had a CCI score >0 at the time of cancer diagnosis. The prevalence of a CCI score ≥3 at the time of diagnosis increased from 8% in 2006–2008 to 12% in 2015–2017 ().
Of the 19 medical conditions in the CCI, the most prevalent were cancers other than RCC (8%), diabetes (8%), cerebrovascular disease (8%), cardiac disease (7%), and chronic pulmonary disease (7%) (Supplementary Table 1).
Survival increased over the four periods of diagnosis, regardless of CCI score (Supplementary Figure 1). Among patients with no CCI comorbidity, 1-year survival increased from 78% for those diagnosed in 2006–2008 to 87% for those diagnosed in 2015–2017, whereas the 5-year survival increased from 57% to 69%. For patients with a CCI score of 1–2, 1-year survival increased from 72% for those diagnosed in 2006–2006 to 86% for those diagnosed in 2015–2017, and 5-year survival increased from 46% to 62%. Survival was lowest among patients with a CCI score ≥ 3, 1-year survival increasing from 71% to 83% and 5-year survival from 39% to 44%. These patterns were similar for patients < 70 years of age and those ≥ 70 years, although survival was highest for patients < 70 years (). Survival decreased with increasing comorbidity scores for patients with both localized/regional and advanced RCC ().
Age- and gender-stratified mortality rates increased with increasing CCI scores. As compared to patients with a CCI score of 0, patients with CCI scores of 1–2 had a slightly higher mortality rate (HR 1.15, 95% CI 1.06–1.24), and the mortality rate of those with a CCI score of ≥ 3 was 1.5 times higher (HR 1.56, 95% CI 1.40–1.73). The patterns were similar for the 5-year mortality rates, whereas only a CCI score ≥ 3 increased the 1-year mortality rate. The patterns were similar for patients < 70 years and ≥ 70 years of age, with mortality rates increase with increased CCI score ().
Table 3. Overall 1– and 5–year survival rates and 95% confidence intervals (CIs) for 7894 patients diagnosed with renal cell carcinoma in Denmark in 2006–2017 by Charlson Comorbidity Index score and stage of the disease.
Table 4. Age- and gender-stratified overall, 1- and 5-year mortality hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) according to Charlson Comorbidity Index score among 7894 patients diagnosed with renal cell carcinoma in Denmark in 2006–2017.
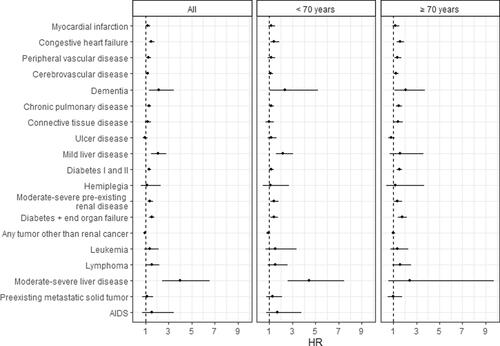
Particularly, diagnoses of liver disease (HR 2.09, 95% CI 1.53–2.84 and HR 4.01, 95% CI 2.44–6.56) and dementia (HR 2.16, 95% CI 1.34–3.48) increased mortality. However, the presence of congestive heart failure, peripheral vascular and cerebrovascular disease, chronic pulmonary disease, preexisting renal disease, diabetes, and lymphoma were also associated with increased mortality (). After mutual adjustment for each of these conditions, the patterns remained the same, although the HRs decreased slightly (data not shown).
Discussion
In this nationwide population-based study, we found that more than one-third of patients had registered comorbidities according to the CCI at the time of RCC diagnosis. The proportions of patients with a high comorbidity burden at the time of diagnosis increased between 2006 and 2017. Although survival increased over time, as also seen in a recent nationwide study [Citation25], the rate was attenuated by increasing comorbidity burden, irrespective of age.
The prevalence of most comorbid conditions at the time of cancer diagnosis was lower in this cohort of patients than in the previous Danish study [Citation8], which may be due to the sources of the cohorts. The previous study included patients from only two of the five Danish health regions, while the present study was nationwide. Thus, geographic inequality in health status may explain some of this discrepancy. Further, in our study, we required that the RCC diagnosis was histologically or cytologically verified, while patients in the previous study were identified only from diagnoses in the Danish National Patient Register. The diagnoses of some patients in the previous study were therefore probably based only on imaging and may have represented older patients with severe comorbidity, who were unfit for further diagnosis and treatment. This assumption is supported by the finding that the median age of patients in the present study was lower than in the previous study. The prevalence of diabetes was higher in the present study than in the previous Danish study (8% versus 6%) [Citation8], probably because, since 2012, diagnosis of diabetes in Denmark has been based on measurements of HbA1c instead of glucose tolerance tests [Citation26]. Furthermore, the prevalence of diabetes in the general population has increased [Citation27].
Comorbidity was also found to be an important prognostic factor for survival among RCC patients in previous studies [Citation7–15]. Most recently, a Korean cohort study showed that the age-adjusted Charlson Comorbidity Index predicted overall survival following nephrectomy among 698 patients with non-metastatic RCC [Citation7]. However, the study comprised a highly selected group of patients eligible for surgery and excluded those with high age or high comorbidity burden. Our study adds further information, as it was a population-based study evaluating the prognostic impact of comorbidity in a population of consecutive patients diagnosed with RCC. Furthermore, we found an association between comorbidity and survival among both younger and older patients with RCC. This is relevant for surgeons when planning optimal treatment of both older and younger patients. Even though RCC survival has increased markedly over time, considerable age-specific relative survival gaps still exist as shown in a recent population-based study on RCC survival in Sweden and Finland [Citation28].
This present study shows that, regardless of the comorbidity burden, survival after RCC has increased considerably over time. This is in line with the results of a recent comparison of survival in the nationwide clinical renal cancer database, DaRenCaData, with that in the Danish Cancer Registry. The study showed that survival after RCC has improved over time, with a 7% yearly reduction in mortality rate [Citation25]. A part of this overall increase in survival is probably due to stage migration, as an increasing number of patients are diagnosed with incidental tumors of earlier stages [Citation1].
The increased survival across CCI groups may reflect improvements in treatment and management of comorbid diseases over time, but also better cancer-related treatment opportunities for patients with comorbidity, due to the use of less invasive surgical procedures like laparoscopic surgery and partial nephrectomy [Citation29].
Regarding the prognostic effect of specific comorbid diseases for survival, we found that congestive heart failure, peripheral vascular and cerebrovascular disease, dementia, chronic pulmonary disease, preexisting renal and liver disease, diabetes as well as lymphoma increased mortality. Similar results were found in a cohort study in the USA of 7177 patients aged > 65 years diagnosed with localized RCC [Citation9]; however, in that study, the highest HRs were found for patients with congestive heart failure and chronic renal disease, whereas liver disease contributed the highest HR in our study. This was due to a high HR for liver disease among patients < 70 years, whereas the US study included only patients > 65 years. Liver disease may be a prominent prognostic risk factor among Danish patients < 70 years because a considerable proportion of liver disease among younger people is probably alcohol-induced and thus associated with an adverse lifestyle and poor health status. After mutual adjustment for all conditions, however, liver disease was still a strong prognostic factor, although the HRs decreased slightly, as for the other conditions.
Strengths and limitations
A major strength of this study is the use of data from nationwide population-based registers with high levels of completeness. This enabled us to establish a nationwide cohort of patients diagnosed with histologically or cytologically verified RCC and to follow them continuously for several years. We, therefore, obtained unique results on the short- and long-term prognostic impact of comorbidity on the survival of patients diagnosed with RCC.
The limitations include lack of information on tumor characteristics, cancer treatment and performance status, which are also important prognostic factors [Citation30]. However, we do not expect tumor characteristics to vary substantially by comorbidity status, and therefore differences in tumor characteristics are unlikely to explain our findings. Cancer treatment and performance status is most likely a part of the causal pathway between comorbidity and survival, and both factors could thus mediate at least some of this association, but would not serve as possible confounders.
Further, as the Danish National Patient Register does not include the diagnoses on diseases that are solely handled among GP’s, there might be an under registration of for instance type 2 diabetes. Most of the diagnoses included in the CCI are, however, so severe that they would lead to a hospital contact at some time in the 10 years before the RCC diagnosis. We, therefore, expect to have captured most of the comorbid conditions [Citation31], although there may have been a minor underestimation of the prevalence of some of the comorbid diseases. We chose to use the CCI to calculate comorbidity. Several other scales with the same purpose are available, for example, the Elixhauser approach, and the National Cancer Institute Index. However, CCI was developed to predict mortality, it has demonstrated a high level of validity and reliability in cancer populations, and it is feasible to use in register-based studies [Citation32].
Survival following RCC is affected by many factors, and the prognostic importance of comorbidity may vary by clinical risk assessment. However, we lacked some of the clinical information needed to compute for example Leibovich's score, and we were therefore not able to assess such variation.
Finally, we studied only overall deaths and were unable to evaluate the effect of comorbidity on RCC-specific mortality.
Conclusions
The survival of patients with RCC has increased over the past decades, regardless of their comorbidity burden. Nevertheless, the prevalence of comorbidity is increasing in this patient group, and this comorbidity affects prognosis, irrespective of age.
Comorbidity should therefore continue to be a focus in treatment planning as well as in the evaluation of the outcome of a renal cell carcinoma diagnosis in patients of all ages.
General data protection regulation
In agreement with the General Data Protection Regulation, the study is registered internally at The Danish Cancer Society (journal number 2019-DCRC-0058).
Supplemental Material
Download MS Word (18.2 KB)Supplemental Material
Download JPEG Image (48.5 KB)Disclosure statement
The authors declare that they have no conflicts of interest.
Data availability statement
The data in this study are accessed through the Danish Health Data Authority. They are not publicly available due to Danish legislation.
Additional information
Funding
References
- Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906.
- Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943–1952.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34.
- Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84.
- Khene ZE, Peyronnet B, Kocher NJ, et al. Predicting morbidity after robotic partial nephrectomy: the effect of tumor, environment, and patient-related factors. Urol Oncol. 2018;36(7):338 e19-33–8 e26.
- Tomaszewski JJ, Uzzo RG, Kutikov A, et al. Assessing the burden of complications after surgery for clinically localized kidney cancer by age and comorbidity status. Urology. 2014;83(4):843–849.
- Kang HW, Kim SM, Kim WT, et al. The age-adjusted Charlson comorbidity index as a predictor of overall survival of surgically treated non-metastatic clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2020;146(1):187–196.
- Lund L, Jacobsen J, Norgaard M, et al. The prognostic impact of comorbidities on renal cancer, 1995 to 2006: a Danish population based study. J Urol. 2009;182(1):35–40.
- Patel HD, Kates M, Pierorazio PM, et al. Comorbidities and causes of death in the management of localized T1a kidney cancer. Int J Urol. 2014;21(11):1086–1092.
- Santos Arrontes D, Fernandez Acenero MJ, Garcia Gonzalez JI, et al. Survival analysis of clear cell renal carcinoma according to the Charlson comorbidity index. J Urol. 2008;179(3):857–861.
- Gettman MT, Boelter CW, Cheville JC, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol. 2003;169(4):1282–1286.
- Berger DA, Megwalu II, Vlahiotis A, et al. Impact of comorbidity on overall survival in patients surgically treated for renal cell carcinoma. Urology. 2008;72(2):359–363.
- Chen DY, Uzzo RG, Viterbo R. Thinking beyond surgery in the management of renal cell carcinoma: the risk to die from renal cell carcinoma and competing risks of death. World J Urol. 2014;32(3):607–613.
- Ather MH, Nazim SM. Impact of Charlson's comorbidity index on overall survival following tumor nephrectomy for renal cell carcinoma. Int Urol Nephrol. 2010;42(2):299–303.
- Kutikov A, Egleston BL, Canter D, et al. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. J Urol. 2012;188(6):2077–2083.
- Erlangsen A, Fedyszyn I. Danish nationwide registers for public health and health-related research. Scand J Public Health. 2015;43(4):333–339.
- Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72–74.
- Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33.
- Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229.
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42–45.
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25.
- Ewertz M, Christensen K, Engholm G, et al. Trends in cancer in the elderly population in Denmark, 1980-2012. Acta Oncol. 2016;55 Suppl 1:1–6.
- Danckert B, Horsbol TA, Andersen O, et al. Registrations of patients with renal cell carcinoma in the nationwide Danish renal cancer database versus the Danish cancer registry: Data quality, completeness and survival (DaRenCa study-3). Clin Epidemiol. 2020;12:807–814.
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus - Abbreviated Report of a WHO Consultation; 2011.
- Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530.
- Hemminki K, Försti A, Hemminki A, et al. Progress in survival in renal cell carcinoma through 50 years evaluated in Finland and Sweden. PLOS One. 2021;16(6):e0253236.
- Lv J, Song R, Cai H, et al. Outcomes of laparoscopic radical nephrectomy for elderly patients with localized renal cell carcinoma. J Buon. 2019;24(5):2147–2154. e0253236.
- Leibovich BC, Lohse CM, Cheville JC, et al. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur Urol. 2018;73(5):772–780.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol. 2012;65(9):924–933.