Abstract
Background
A systematic assessment of problems that are frequent in older age (geriatric assessment [GA]) provides prognostic information for patients undergoing cancer surgery and systemic cancer treatment. We aimed to investigate the prevalence of geriatric impairments and their impact on survival in older patients with cancer receiving radiotherapy (RT).
Material and methods
A single-centre prospective observational study was conducted including patients ≥65 years referred for curative or palliative RT. Prior to RT, we performed a modified GA (mGA) assessing comorbidities, medications, nutritional status basic- and instrumental activities of daily living (IADL) mobility, falls, cognition and depressive symptoms. Impairments in each mGA domain were defined. Overall survival (OS) was presented by Kaplan Meier plots for groups defined according to the number of impairments, and compared by log-rank test. The association between mGA domains and OS was assessed by Cox proportional hazard regression analysis.
Results
Between February 2017 and July 2018, 301 patients were included, 142 (47.2%) were women. Mean age was 73.6 (SD 6.3) years, 162 (53.8%) received curative RT. During the median observation time of 24.2 months (min 0.3, max 25.9), 123 (40.9%) patients died. In the overall cohort, 49 (16.3%) patients had no geriatric impairment, 81 (26.9%) had four or more. OS significantly decreased with an increasing number of impairments (p < .01). Nutritional status (HR 0.90, 95% CI [0.81; 0.99], p = .038) and IADL function (HR 0.98, 95% CI [0.95; 1.00], p = .027) were independent predictors of OS.
Conclusion
Geriatric impairments were frequent among older patients with cancer receiving RT and nutritional status and IADL function predicted OS. Targeted interventions to remediate modifiable impairments may have the potential to improve OS.
Trial registration
Cinicaltrials.gov ID:NCT03071640.
Background
The number of patients with cancer who are 65 years or older is rapidly increasing and constitutes the majority of patients with cancer [Citation1,Citation2]. Despite this, older patients are still underrepresented in clinical cancer trials [Citation3–6]. The evidence base for optimal care of these patients is thus limited. In general, older patients have a higher risk of negative treatment outcomes than their younger counterparts. However, health status and thereby treatment tolerance may vary considerably. Treatment decisions based on chronological age alone or simple assessment tools such as Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) may result in both over- and undertreatment [Citation7–9]. More detailed assessments covering overall health status are needed to appropriately individualise treatment and follow-up [Citation6], and for this, a geriatric assessment (GA) is widely recommended [Citation8,Citation10].
GA is a systematic evaluation of domains where older patients commonly have a problem and usually includes physical health (comorbidities, medications, and nutritional status), functional status (activities of daily living), psychological status (cognitive and emotional) and socio-environmental factors [Citation11]. Increasing evidence suggests that GA captures important information that is otherwise lost in ordinary oncology consultations [Citation12], and that has direct therapeutic consequences [Citation13]. Furthermore, GA has been shown to be predictive of outcomes for older patients with cancer receiving surgery or systemic treatment, including postoperative complications [Citation14], chemotherapy toxicity [Citation15] and survival [Citation16]. The extent to which GA may predict survival and other outcomes in a radiotherapy setting is still scarcely documented [Citation9,Citation16–18].
Radiotherapy (RT) is a cornerstone in both curative and palliative cancer treatment and is estimated to be needed by 45-60% of patients during the course of their disease [Citation19,Citation20]. Although RT is generally regarded as more tolerable than most cancer surgery and systemic cancer treatment, it may have serious short- and long-term side effects [Citation18]. Moreover, most established RT regimes involve daily treatment for several days or even weeks, which can be an additional burden, particularly for patients travelling long distances. Older patients with reduced resilience to stressors are most prone to these negative consequences [Citation18]. When treating older patients, it is not only paramount to weigh the total burden of treatment against the benefits, but also to assess if the patient will live long enough to experience these benefits. Given that impairments in the domains covered by GA affect prognosis in other oncologic treatment settings [Citation21], it is essential to discover their potential impact on older patients’ survival after RT. In this study, we assessed a non-selected population of patients with cancer aged ≥65 years who were receiving RT with palliative or curative treatment intent. We aimed to answer the following questions: 1) How frequent are geriatric impairments identified by a GA? And 2) do impairments in GA domains predict survival in patients receiving RT?
Material and methods
Setting and patients
We conducted a prospective single-centre observational study at the Radiotherapy Unit (RTU), Innlandet Hospital Trust. The RTU serves a catchment area of approximately 370 000 inhabitants [Citation22], and provides external beam RT delivered according to modern techniques including intensity-modulated RT (IMRT). RT with palliative intent is offered to all cancer groups, whilst potentially curative RT is only given to selected diagnoses, including breast, prostate, lung, and some skin cancers. Candidates for curative RT with other diagnoses or for specific RT techniques, for instance stereotactic RT, are referred to Oslo University Hospital. Patients were consecutively recruited from February 2017 to July 2018. The eligibility criteria were: age ≥65 years, referred for curative or palliative RT, resident of Innlandet County, histologically verified cancer diagnosis, fluent in oral and written Norwegian, and able to answer self-report questionnaires.
Assessments
Sociodemographic data were obtained through patient interviews. Medical data were retrieved from the patients’ electronic medical record (EMR) and the treating radiation oncologist. RT treatment intent, curation or palliation, was registered as defined by the treating radiation oncologist in patients’ EMR. ECOG PS was dichotomised as 0–1 and 2–4. Cancer diagnoses were divided into the following groups: 1) breast, 2) prostate, 3) lung and 4) other types of cancer. All patients underwent baseline GA prior to RT. The procedure was performed by a study nurse and a resident oncologist (both specifically trained), not a geriatric team [Citation23], thus further referred to as a modified GA (mGA). The mGA comprised nine domains which were all assessed by validated tools and questionnaires. Geriatric impairments within each domain were retrospectively defined in accordance with formerly used and/or well-established cut-off values (). Comorbidities were registered using the ICD-10 version of Charlson Comorbidity Index (CCI) [Citation24,Citation25], not age-adjusted (scores 0-26), and based on information from patients and their EMR. Prescribed regular medications were registered according to the Anatomical Therapeutic Chemical (ATC) Classification System. Nutritional status was assessed by the Mini Nutritional Assessment Short Form (MNA-SF) [Citation26], (scores 0-14, higher scores indicate better status) [Citation27]. Mobility was tested by ‘Timed Up and Go’ (TUG) [Citation28], counting mean seconds of two repetitions performed at normal pace. Patients reported the number of falls during the past six months (dichotomised as 0-1 fall or ≥2 falls). Basic activities of daily living (ADL) (self-care) and instrumental ADL (IADL) (e.g., housekeeping, managing transportation and medications) were assessed by patients’ self-report using Barthel Index (scores 0-22) [Citation29] and Nottingham Extended Activities of Daily Living (NEADL)(scores 0-66) [Citation30]. Higher scores indicate better function. Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) test [Citation31] (scores 0-30, higher scores indicating better cognition), and patients with educational level ≤ 12 years were assigned one point extra [Citation31]. Patients answered Geriatric Depression Scale-15 (GDS-15) [Citation32] to reveal depressive symptoms (scores 0-15, higher scores indicate more symptoms). Polypharmacy was defined as ≥5 regular prescribed medications [Citation33,Citation34]. MoCA scores are age-dependent [Citation35], thus, leaning on age-specific strata from Swedish normative data [Citation35], we defined cut-off values for cognitive impairment in two age groups 1) 65–75 years ≤23 and 2) >75 years ≤21 (). Patients were followed for maximum 2 years after completing RT or until death. Information about survival was retrieved from patients’ EMR.
Table 1. Domains in the modified geriatric assessment (mGA) and definitions of impairments.
Statistical analyses
The primary outcome was overall survival (OS) defined as time from inclusion to death, or to last observation date, 21 April 2020. Sociodemographic and medical data were described as means and standard deviations (SD) or median and min–max values for continuous variables, and as frequencies and percentages for categorical variables. Comparison of mGA results between patients receiving RT with curative or palliative treatment intent was performed by independent samples t-test or χ2-test, as appropriate. The prevalence of impairments in all mGA domains was estimated based on the defined cut-off values. Missing single values in MoCA (n = 1), Barthel index (n = 6) and NEADL (n = 20) were imputed if at least half the scale had been answered. The imputation was performed by generating an empirical distribution for each item based on non-missing values, and a random number drawn from it was used to replace the missing value. Six patients were excluded from the regression analyses due to missing MoCA (n = 3) and missing NEADL scores (n = 3), resulting in n = 295. TUG was missing for 19 patients who were unable to perform the test. To account for this in the regression analyses TUG values of all patients were inverted and patients missing TUG were assigned the value 0 (i.e., indefinitely long time). A higher value of the inverted TUG thus means shorter time performing the test. OS was illustrated by Kaplan-Meier curves and was compared between patients receiving curative or palliative RT by a log-rank test. For explorative purposes, differences in survival between groups defined according to number of mGA impairments (categorised 0, 1, 2, 3, ≥4) were also assessed. Unadjusted and adjusted Cox proportional hazard regression models were estimated to assess the impact of mGA domains (CCI, medications, MNA-SF, TUG, falls, NEADL, MoCA and GDS-15) on OS. The models were adjusted for: age, gender, type of cancer (diagnosis group) and treatment intent. Correlation analysis (not shown) between mGA domains, the possible confounders and ECOG PS, was performed a priori to regression analyses to assess potential multicollinearity issues. Due to high correlations between variables (TUG, NEADL, Barthel Index and ECOG PS), Barthel Index and ECOG PS were considered but not included in the adjusted regression model. The interactions between treatment intent and all other variables in the model were included. The Akaike’s Information Criterion (AIC), where the smaller value means a better model, was applied to reduce the model for excessive interactions. Proportional hazards assumption was tested by assessing Schoenfeld’s residuals. As ECOG PS had to be excluded from the main adjusted regression model as described, we explored whether this variable could predict OS as a proxy to performing mGA. All mGA domains were thus substituted for ECOG PS in an explorative Cox regression model adjusted for the same factors as our main model. We compared the two adjusted models with a C-index. All tests were two-sided, and results with p-values below 0.05 were considered statistically significant. Statistical analyses were performed in SPSS Statistics 26 and Stata version 16.
Ethics and approval
Patients provided written informed consent. The treating radiation oncologist was blinded to the mGA results. However, before study recruitment started, a manual was made providing advice on further actions if previously unrecognised severe geriatric problems were revealed through baseline assessments. The study was approved by the Regional Committee for Medical Research Ethics South East Norway and was registered at clinicaltrials.gov (NCT03071640).
Results
Study recruitment and patients characteristics
During the recruitment period 538 patients ≥65 years referred for RT were screened for inclusion and 509 were eligible. Among these 148 (29.1%) declined to participate, 28 (5.5%) were considered too sick, and 32 (6.3%) were not included for other reasons (e.g., change of treatment plan, study nurse absent). Therefore, 301 (59.1%) patients were enrolled, 142 (47.2%) were women, and mean age was 73.6 (SD 6.3) years (). RT with curative intent was given to 162 (54%) patients, while 139 (46%) received palliative RT. In comparison with patients receiving palliative RT, the curative group included more women (56.8 vs 36.0%), more breast (50.6 vs 9.4%) and prostate cancer patients (31.5 vs 15.8%), fewer lung (9.3 vs 36.0%) and ‘other types of cancer’ (8.6 vs 38.8%), and more PS 0-1 patients (95.7 vs 72.7%) (). In the overall cohort, 49 (16.3%) patients had no geriatric impairment, whereas 81 (26.9%) had four impairments or more (). Compromised nutritional status (MNA-SF) (55.5%), polypharmacy (55.1%) and reduced cognition (MoCA) (33.3% aged 65–75 years, and 39.0% age >75 years) were most prevalent. Physical and functional impairments were also frequent, i.e., for TUG 21.3%, ≥2 falls 11.3%, NEADL 21.1% and Barthel 19.9%, and 20.9% reported depressive symptoms (GDS ≥ 5). Comparing the curative and the palliative group, the latter had poorer average scores in all mGA domains, and, except for falls, the differences were statistically significant. The proportion of impairments was also higher in the palliative group ().
Table 2. Baseline patient characteristics.
Table 3. Mean scores in mGA domains and proportions of patients with geriatric impairments according to treatment intent.
Survival
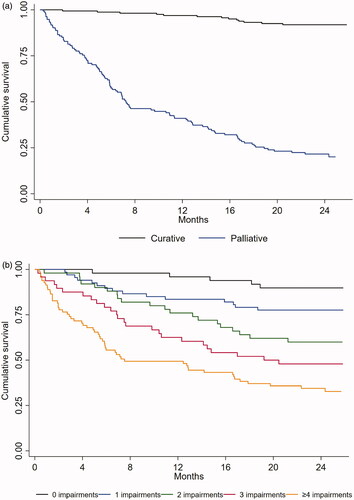
Median observation time was 24.2 months (min 0.3, max 25.9). During this period 123 (40.9%) patients died, 13 (8.0%) in the curative group and 110 (79.1%) in the palliative group (). Within 1 month, 3 months and 1 year after RT, one (0.6%), one (0.6%) and six (3.7%) patients in the curative group had died, while this occurred in 18 (12.9%), 33 (23.7%) and 55 (60.4%) in the palliative group. The cumulative survival probability for the entire cohort was 93.7% at 1 month, 88.7% at 3 months, 70.1% at 1 year, and 59.1% 2 years after RT. Median OS was not reached, but for all patients mean OS was 18.5 months (95% CI [17.4; 19.6]).Mean OS for curative patients was 24.8 months (95% CI [24.2; 25.4]), and for palliative patients 11.0 months (95% CI [9.5; 12.5], p = .001) (). Explorative analysis showed a significant difference in OS between patients having no, one, two, three, or four and more geriatric impairments (p < .001) (), and that OS decreased with an increasing number of impairments.
Factors predictive of survival
According to unadjusted Cox regression analyses (), all mGA domains, with the exception of falls, were significant prognostic factors for OS, among both curative and palliative patients. According to the adjusted model, MNA-SF (HR 0.90, 95% CI [0.81; 0.99], p = .038) and NEADL (HR 0.98, 95% [CI 0.95; 1.00], p = .027) remained significant (). A one-unit increase in MNA-SF score reduced the risk of death with 10%, whereas a five-unit increase reduced the risk with 42% (HR 0.58). For the NEADL scores (range 0-66), a one- and a ten-unit increase reduced the mortality risk with 2% and 22% (HR 0.78), respectively. We identified a significant interaction between cancer type and treatment intent. Post hoc analyses of this interaction term showed that, among curative patients, those having lung- or other type of cancer had a significantly higher risk of dying than breast and prostate cancer patients. Among palliative patients, only prostate cancer was associated with a higher mortality than lung cancer, with no other differences between cancer groups. In our explorative regression analyses (), we found that having ECOG PS score 2-4 was strongly associated with reduced survival both according to the unadjusted model (HR 3.70, 95% CI [2.47; 5.53], p˂0.001), and the model adjusted for age, gender, diagnosis and treatment intent (HR 1.71, 95% CI [1.12; 2.62], p = .012). The C-index for this model was 0.843 (95% CI [0.812; 0.874]) compared to 0.867 (95% CI [0.840; 0.893], p = .033) for the main model including all mGA domains.
Table 4. Cox regression analyses of the association between mGA domains and overall survival, N = 295.
Table 5. Explorative cox regression analyses of the association between ECOG PS and overall survival, N = 295.
Discussion
In this observational study older patients with cancer were assessed with mGA before receiving either curative or palliative RT. We found an overall high prevalence of geriatric impairments, but for all mGA domains, deficits were most frequent in the group referred to palliative RT. Better nutritional status and IADL function were associated with prolonged OS, independent of age, gender, diagnoses and treatment intent.
Only a minority of our patients had no geriatric impairments, whereas 44% in the curative group and 78% in the palliative group had two or more, a number frequently used to define frailty [Citation3]. Frailty is recognised as a syndrome of increased vulnerability to stressors, and has a well-documented association with adverse outcomes [Citation3,Citation40]. In older patients with cancer median prevalence of frailty is reportedly about 40% [Citation3] placing our RT cohort, and in particular the palliative patients, among the most affected. Moreover, we found that compromised nutritional status and polypharmacy were the most common geriatric impairments, which is in line with findings of a systematic review [Citation13]. Comparisons between studies, are, however, dubious as prevalence of impairments is likely to vary with characteristics of the target population, assessment methods and cut-off values.
To the best of our knowledge, no former prospective study has investigated the predictive effect of individual GA domains on survival in older patients receiving RT. Our unadjusted analyses demonstrated a clear association between all mGA domains (except falls) and survival, and this association was further underlined by the distinct difference in survival between groups defined according to number of geriatric impairments. However, the only mGA domains that were independently predictive of survival in our cohort was nutritional status and IADL. The former is supported by a range of studies from other cancer settings [Citation16,Citation41], as well as a study on patients ≥ 60 years receiving RT for oesophagus cancer [Citation42]. The importance of pre-treatment IADL function for survival has also formerly been reported in older patients receiving other cancer treatment [Citation16,Citation43]), a knowledge hereby expanded to those treated with both palliative or curative RT.
The prevalence and impact of geriatric impairments documented in this study, underline the utility of implementing existing recommendations for GA into radiation oncology practice [Citation9,Citation17]. First of all, GA domains and the number of geriatric impairments hold important prognostic information, and, as suggested by our explorative analyses, using mGA domains to predict survival is likely to be more precise than relying on ECOG PS alone. This finding is supported by previous research emphasising the additional prognostic information provided by GA [Citation7,Citation12], and that ECOG PS has limitations in the assessment of older patients [Citation7,Citation15]. To estimate prognosis is essential for treatment planning and choice of treatment regimens. In the RT setting, uncovering geriatric impairments that indicate poor prognosis, may allow the radiation oncologist to consider alternatives to conventional RT regimen that are less time consuming and may be less burdensome, such as hypofractioned or stereotactic radiation [Citation18]. Furthermore, for some of the frailest patients the omission of RT and providing best supportive or palliative care may be a better option. Secondly, recognising geriatric impairments is essential to implementing targeted supportive interventions. Nutritional status and IADL function, which according to our findings may have the most pronounced negative effect on survival, are potentially remediable. Malnutrition can be part of older adults’ intrinsic vulnerability, a problem that could be reinforced by a cancer diagnosis. Although reversing the catabolic state associated with malignant disease may not be possible, focus on alleviating symptoms such as nausea, nutritional counselling and correction of underlying causes, e.g. inappropriate polypharmacy or sore mouth, may improve nutritional status for many patients [Citation41]. Pre- and rehabilitation together with adequate treatment of symptoms such as pain, can improve functional status and promote IADL independency [Citation44]. Appropriate attention to the other frequent impairments in our cohort, i.e., depressive symptoms, cognitive dysfunction and ADL deficits, is also likely to minimise the patients’ burden and improve outcomes. Ideally, GA should be performed upon referral to RT so that interventions aiming to remediate impairments can start prior to RT and continue during and after treatment [Citation10]. These efforts could help avoiding overtreatment, making personalised treatment adaptations and implementing non-oncologic measures to promote the patient’s treatment tolerance [Citation45]. Although the potential benefits of GA followed by targeted management of impairments are hitherto poorly documented in older patients with cancer, the frequency and complexity of geriatric problems as shown in this study emphasise the importance of a multidisciplinary approach to older patients receiving RT.
The strengths of this study are the prospective design, the relatively large sample size and that no patients were lost to follow-up. Furthermore, except for social support, our mGA incorporated all recommended domains [Citation8,Citation11], and validated test and methods were used., The assessments were performed by only two equally trained professionals, and to define impairments we used formerly reported and validated cut-offs whenever possible.
Our study has several limitations. Using broad inclusion criteria, we aimed to address a non-selected older population representative of patients seen on a daily basis at an RTU. Thus, our cohort is heterogeneous and OS may have been affected by several confounding factors, not accounted for in our analyses. Furthermore, curative treatment on the study site was restricted to selected diagnoses, and only 59.1% of eligible patients were included. This limits the generalisability, and as the majority of non-included patients were either too sick or declined to participate, it is possible that our patients may represent the fittest part of the older RT population. It also raises the question of feasibility since GA may be considered too demanding both from the patients’ and the health care providers’ perspective. In the RT setting a two-step model, where a brief frailty screening tool is applied before proceeding to a comprehensive GA if indicated, may be easier to implement [Citation46]. A final objection may be that ECOG PS was excluded from the main regression model. As explained, this was due to multicollinearity. We therefore investigated the predictive effect of ECOG PS in an exploratory model, and found that a model considering mGA domains was superior in predicting OS. Representing an exploratory analysis this finding should, however, be interpreted with caution.
In conclusion, we found that geriatric impairments identified by GA are frequent and provide important prognostic information about older patients with cancer receiving RT. Implementing GA into radiation oncology practice has the potential to facilitate decision-making and improve outcomes and care for the increasing number of older patients with complex health issues. However, further research, preferably randomised controlled trials, investigating the effect of GA-based tailored interventions on survival and other outcomes of RT are warranted.
Acknowledgement
The authors are very thankful to all patients who participated in this study and to Bodil Sem Kolsgaard, the study nurse who interviewed the vast majority of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Norway CRO. Cancer in Norway 2017 - cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2018.
- UK Cr. Cancer incidence by age [updated 20.032020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age#heading-Zero.
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101.
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067.
- Hurria A, Dale W, Mooney M, Cancer and Aging Research Group, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32(24):2587–2594.
- Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5(12):1769.
- Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern cooperative oncology group performance status in elderly cancer patients: an italian group for geriatric oncology study. J Clin Oncol. 2002;20(2):494–502.
- Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347.
- Popescu T, Karlsson U, Vinh-Hung V, et al. Challenges facing radiation oncologists in the management of older cancer patients: Consensus of the international geriatric radiotherapy group. Cancers (Basel). 2019;11(3):371.
- Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603.
- O'Donovan A, Mohile SG, Leech M. Expert consensus panel guidelines on geriatric assessment in oncology. Eur J Cancer Care (Engl)). 2015;24(4):574–589.
- Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120–132.
- Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients-a systematic review. Acta Oncol. 2014;53(3):289–296.
- Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76(3):208–217.
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465.
- Hamaker ME, Vos AG, Smorenburg CH, et al. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439–1449.
- Szumacher E, Sattar S, Neve M, et al. Use of comprehensive geriatric assessment and geriatric screening for older adults in the radiation oncology setting: a systematic review. Clin Oncol (R Coll Radiol)). 2018;30(9):578–588.
- O'Donovan A, Leech M, Gillham C. Assessment and management of radiotherapy induced toxicity in older patients. J Geriatr Oncol. 2017;8(6):421–427.
- Slotman BJ, Cottier B, Bentzen SM, et al. Overview of national guidelines for infrastructure and staffing of radiotherapy. ESTRO-QUARTS: work package 1. Radiother Oncol. 2005;75(3):349–354.
- Somasundar P, Mourey L, Lozza L, et al. Advances in geriatric oncology: a multidisciplinary perspective. Tumori. 2018;104(4):252–257.
- Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, et al. Predictive value of each geriatric assessment domain for older patients with cancer: a systematic review. J Geriatr Oncol. 2019;10(6):859–873.
- fylkeskommune I. Fakta om Innlandet 2020. [updated 19.06.2020]. Available from: https://innlandetfylke.no/om-fylkeskommunen/om-innlandet/fakta-om-innlandet/.
- Puts MTE, Alibhai SMH. Fighting back against the dilution of the comprehensive geriatric assessment. J Geriatr Oncol. 2018;9(1):3–5.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294.
- Rubenstein LZ, Harker JO, Salva A, et al. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. 2001;56(6):M366–72.
- Kaiser MJ, Bauer JM, Ramsch C, MNA-International Group, et al. Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–788.
- Podsiadlo D, Richardson S. The timed "up & go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148.
- Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65.
- Gladman JR, Lincoln NB, Adams SA. Use of the extended ADL scale with stroke patients. Age Ageing. 1993;22(6):419–424.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. 1986;5(1-2):165–173.
- Mohile SG, Epstein RM, Hurria A, et al. Communication with older patients with cancer using geriatric assessment: a Cluster-Randomized clinical trial from the national cancer institute community oncology research program. JAMA Oncol. 2020;6(2):196–204.
- Gutiérrez-Valencia M, Izquierdo M, Cesari M, et al. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84(7):1432–1444.
- Borland E, Nägga K, Nilsson PM, et al. The montreal cognitive assessment: Normative data from a large swedish Population-Based cohort. JAD. 2017;59(3):893–901.
- Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385.
- Owusu C, Koroukian SM, Schluchter M, et al. Screening older cancer patients for a comprehensive geriatric assessment: a comparison of three instruments. J Geriatr Oncol. 2011;2(2):121–129.
- Kristjansson SR, Jordhøy MS, Nesbakken A, et al. Which elements of a comprehensive geriatric assessment (CGA) predict post-operative complications and early mortality after colorectal cancer surgery? Journal of Geriatric Oncology. 2010;1(2):57–65.
- Pocklington C, Gilbody S, Manea L, et al. The diagnostic accuracy of brief versions of the geriatric depression scale: a systematic review and Meta-analysis. Int J Geriatr Psychiatry. 2016;31(8):837–857.
- Vermeiren S, Vella-Azzopardi R, Beckwée D, Gerontopole Brussels Study group, et al. Frailty and the prediction of negative health outcomes: a Meta-Analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–1163.e17.
- Hamaker ME, Oosterlaan F, van Huis LH, et al. Nutritional status and interventions for patients with cancer - A systematic review. J Geriatr Oncol. 2021:12(1):6–21.
- Bo Y, Wang K, Liu Y, et al. The geriatric nutritional risk index predicts survival in elderly esophageal squamous cell carcinoma patients with radiotherapy. PLoS One. 2016;11(5):e0155903.
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007 May 10;25(14):1824–1831.
- Connolly D, Garvey J, McKee G. Factors associated with ADL/IADL disability in community dwelling older adults in the irish longitudinal study on ageing (TILDA). Disabil Rehabil. 2017;39(8):809–816.
- Rostoft S, O'Donovan A, Soubeyran P, et al. Geriatric assessment and management in cancer. J Clin Oncol. 2021;39(19):2058–2067.
- O'Donovan A, Leech M. Personalised treatment for older adults with cancer: the role of frailty assessment. Tech Innov Patient Support Radiat Oncol. 2020;16:30–38.