Abstract
Background
Lung cancer is the number one cancer-related cause of death in Sweden and worldwide. In most countries, five-year survival estimates vary between 10% and 20% with evidence of improved survival over time. Over the last decades, the management of lung cancer has changed including the introduction of national guidelines, new diagnostic procedures and treatments. This study aimed to investigate temporal trends in lung cancer survival both overall and in subgroups defined by established prognostic factors (i.e., sex, stage, histopathology and smoking history).
Materials and methods
We estimated one-, two-, and five-year relative survival, and excess mortality, in patients diagnosed with squamous cell carcinoma or adenocarcinoma of the lung between 1995 and 2016 in Sweden. We used population-based information available in a national lung cancer research database (LCBaSe) generated by cross-linkage between the Swedish National Lung Cancer Register and several Swedish health and sociodemographic registers.
Results
We included 36,935 patients diagnosed with squamous cell carcinoma or adenocarcinoma of the lung between 1995 and 2016. The overall one-, two- and five-year survival estimates increased between 1995 and 2016, from 38% to 53%, 21% to 37%, and 14% to 24%, respectively. Over the study period, we also found improved survival in subgroups, for example in patients with stages III-IV disease, patients with adenocarcinoma, and never-smokers. The excess mortality decreased over the study period, both overall and in all subgroups.
Conclusion
Lung cancer survival increased over time in the overall lung cancer population. Of special note was evidence of improved survival in patients with stage IV disease. Our results corroborate a previously observed global trend of improved survival in patients with lung cancer.
Introduction
The prognosis for patients with lung cancer remains poor and lung cancer is the most common cancer-related cause of death in Sweden and worldwide [Citation1–3].
Over the past few decades, there have been gradual changes in the management of lung cancer including the introduction of national guidelines and policies, improvements in diagnostic procedures and the introduction of new treatments [Citation4,Citation5]. While these changes have affected the management of all patients with lung cancer, it is unclear to what extent they have affected outcomes in subgroups defined by sex, cancer stage, histopathology and smoking history.
To date, there are only a few large population-based studies on routinely collected healthcare data, which in detail have estimated trends in lung cancer survival over the past 20 years, both overall and in subgroups.
This population-based study aimed to investigate temporal trends in lung cancer survival by use of routinely collected health care data in the setting of a tax-funded national health care system.
Materials and methods
Study design and study population
We conducted a population-based study to investigate temporal trends in relative survival among patients diagnosed with lung cancer (International Classification of Diseases 7th revision [ICD–7] code: 1621 or ICD for oncology 3rd revision [ICD–O–3] code: C34) of squamous cell or adenocarcinoma histopathology in Sweden between 1995 and 2016.
Data sources and variables
For the purpose of the present study, we used data from the Lung Cancer Database Sweden (LCBaSe) [Citation6–8], a research database generated by cross-linkage between the Swedish National Lung Cancer Register (NLCR) and several Swedish health registers: the Swedish Cancer Register, the National Patient Register, the Prescribed Drug Register, the Cause of Death Register, the Multi-Generation Register, the Swedish Population Register, and the LISA–database (a database incorporating sociodemographic data from various nationwide population-based registers) [Citation9–16]. The linkage was made possible by the use of the unique personal identification number assigned to all residents in Sweden [Citation17].
The NLCR aims to include all newly diagnosed patients with lung cancer in Sweden [Citation9]. Between 1995 and 2001, this was a regional population-based register covering the Uppsala–Örebro healthcare region in Central Sweden (approximately two million inhabitants, 20% of the Swedish population). Compared to the Swedish Cancer Register, to which reporting is mandated by law, the NLCR includes 97% of newly diagnosed patients with lung cancer [Citation4,Citation10]. In addition, the NLCR includes information of high validity on smoking history and stage [Citation4,Citation10,Citation18]. From the same source, we used data on a calendar year of diagnosis (1995–2016), sex (women, men), age in years (<50, 50–59, 60–69, 70–79, and ≥80), smoking history (based on self–reported information from the patient to the physician; current smokers, former smokers, never-smokers), histopathology (squamous cell carcinoma, adenocarcinoma) and cancer stage (based on the tumour–node–metastasis [TNM] classification system by the American Joint Committee on Cancer; I-II, III, IV) as recorded at the time of diagnosis. Information on the date of death and date of emigration was obtained from the Swedish Population Register [Citation15].
Statistical methods and analysis
Descriptive statistics were used to describe clinical characteristics and demographics, summarised by counts and percent for categorical variables, or median with first quartile (q1) and third quartile (q3) for continuous variables.
Relative survival, the ratio of the observed survival for patients with lung cancer to the expected survival had they been free of lung cancer [Citation19], was estimated for separate calendar years of diagnosis at one, two and five years post-diagnosis. This was performed overall and in subgroups defined by sex, cancer stage, histopathology and smoking history. Survival time for patients was counted from the date of the lung cancer diagnosis until the date of death, emigration, or end of the study period (i.e., administrative censoring, 31 December 2016), whichever occurred first. We excluded patients with a negative follow-up time. A negative follow-up time arises when the recorded date of death precedes the date of diagnosis. This may for example be because of incorrect registration of the date of death or the date of diagnosis in the data sources. The expected survival was calculated with the Ederer II estimator derived from the Swedish general population using the life table approach. The life tables were downloaded from the Human Mortality Database, and stratified by calendar year, sex and age [Citation20]. To estimate relative survival using the cohort approach, all patients were required to have a potential follow–up of at least that number of years, for example, if estimating five-year relative survival the patients were required to have a potential follow–up of at least five years [Citation19]. For the more recent years under study, it was not possible to use the cohort approach; instead, the period approach was used (Supplementary Figure 1). In the period approach, all observations included in the analysis were left–truncated at the beginning and right–truncated at the end of the study period [Citation19]. To address changes in the age distribution of included patients over the study period and to be able to compare our results to those from other countries, the relative survival estimates were age-standardised according to the first International Cancer Survival Standard [Citation21].
Excess mortality was modelled using Poisson regression models adjusted for age and sex, with the last calendar period (2012–2016) as the reference period [Citation19]. P-values for the trend of the excess mortality over calendar periods were assessed assuming a linear trend. We also evaluated the relationship assuming non-linear trends, for example, quadratic and cubic, however, they did not manage to describe the data in a significantly better way than the linear trend. To assess if the effect of calendar period varied across subgroups of a characteristic (i.e., sex, cancer stage, histopathology or smoking history), p-values for interaction were estimated using likelihood ratio tests comparing a Poisson model with an interaction term between calendar period and the characteristics of interest to a model without the interaction term. The strs command in Stata was used to estimate relative survival and to model excess mortality. Missing information in variables was handled as a separate category in the variable.
Stata version 16.1 and R statistical packages version 4.0.0 [Citation22,Citation23], or later versions, were used for all data management and statistical analyses.
Ethical approval
This study was approved by the Regional Ethical Review Boards in Uppsala and Stockholm (record number: 2012/1162–31/4, 2016/1137–32, 2017/445–32, 2017/2026-32).
Results
Descriptive analysis
A total of 37,166 patients diagnosed with squamous cell carcinoma or adenocarcinoma of the lung between 1995 and 2016 were identified in the NLCR. Of these, 231 were excluded because of a negative follow-up time. Thus, the final study population was comprised of 36,935 individuals (). The absolute annual number of patients diagnosed with lung cancer increased over the study period. In 2002, 1,497 patients were recorded as having a diagnosis of squamous cell carcinoma or adenocarcinoma of the lung in the NLCR, compared to 2,989 in 2016.
Table 1. Baseline characteristics of patients diagnosed with adenocarcinoma or squamous cell carcinoma of the lung, Lung Cancer Data Base Sweden, 1995–2016.
Of the included patients, 48% were women and 52% were men. The median age at diagnosis was 70 years (q1: 63, q3: 76). Stage IV was the most common stage at diagnosis with the proportion of patients with stage IV disease increasing from 36% in 1995–2001 to 51% in 2012–2016. The proportion of adenocarcinomas increased from 54% to 74% over the study period. The proportion of never–smokers remained stable at approximately 10% during the study period.
Relative survival and excess mortality
We found that the age-standardised relative survival for the whole cohort improved between 1995 and 2016 (, , Supplementary Table 1). The one-year survival estimate increased from 38% to 53%, the two-year survival estimate increased from 21% to 37%, and the five-year survival estimate increased from 14% to 24%. Only small differences in point estimates between un-standardised and age-standardised relative survival estimates for the overall cohort were found, and generally, they followed a similar temporal trend in relative survival (Supplementary Figure 2, Supplementary Table 2). In addition, we found that the age-standardised relative survival improved in subgroups of patients with lung cancer (, Supplementary Figures 3–6, Supplementary Tables 3–6), for example, for patients with stages III-IV disease, patients with adenocarcinoma, and for never-smokers.
Figure 1. Age-standardised one-, two- and five-year relative survival estimates over calendar years overall in patients diagnosed with adenocarcinoma or squamous cell carcinoma of the lung, Lung Cancer Data Base Sweden, 1995–2016. Note: The years 1995–2001 include only the Uppsala-Örebro Health Care Region (approximately 2 million inhabitants, 20% of the Swedish population) 1-year: 38% (1995) – 53% (2016); 2-year: 21% (1995) – 37% (2016); 5-year: 14% (1995) – 24% (2016)
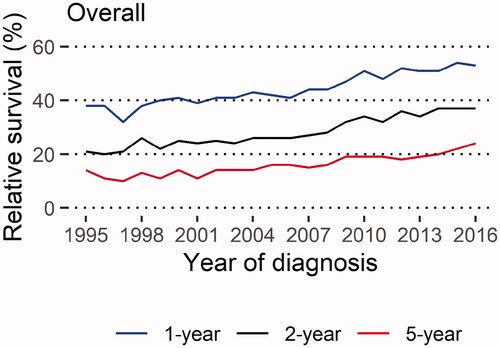
Table 2. Absolute change in a point estimate for age-adjusted relative survival (RS) in patients diagnosed with adenocarcinoma or squamous cell carcinoma of the lung, Lung Cancer Data Base Sweden, 1995–2016.
Excess mortality, adjusted for age and sex, decreased over the study period both overall and in all subgroups (). There was no difference between men and women in the decrease of excess mortality. The decrease in excess mortality was greater for patients with non-distant metastatic cancer compared to patients with distant metastases. Similar, the decrease was greater for patients with adenocarcinoma compared to patients with squamous cell carcinoma, and for never-smokers compared to ever-smokers.
Table 3. Excess mortality ratio, adjusted for sex and age, over calendar periods in patients diagnosed with adenocarcinoma or squamous cell carcinoma of the lung, Lung Cancer Data Base Sweden, 1995–2016.
Discussion
Main findings
To the best of our knowledge, this is one of the largest nationwide studies based on routinely collected data that assess long–term temporal trends in lung cancer survival in the setting of a national tax-funded health care system, overall and by prognostic subgroups. The age-standardised relative survival improved, and the excess mortality decreased, between 1995 and 2016.
Interpretation and comparison to other studies
Improvements in the prognosis of patients with lung cancer have also been observed in other countries and health care settings [Citation3,Citation24], including Norway and Estonia [Citation25–27]. The improved survival in our study is also reflected by the decreases in excess mortality. Our findings of a decrease in excess mortality corroborate results from studies from other European countries [Citation26,Citation27], and observations of a long-term decreasing trend in excess mortality in the Swedish lung cancer population [Citation28].
Our findings of an overall improvement in lung cancer survival are likely to reflect a combination of changes occurring in parallel between 1995 and 2016, and gradually across regions of Sweden. These changes include the implementation of uniform national guidelines and policies, continuous monitoring of the quality of care by use of data in the NLCR, resource allocation, and the introduction of new diagnostic procedures and therapies [Citation4,Citation5,Citation9]. In addition, changes over time in lifestyle factors and comorbidity burden are also likely to have contributed to improved outcomes [Citation29].
National guidelines and policies
The first common recommendations for the management of patients with lung cancer in Sweden were introduced in the early 1990s and the first national uniform guideline on lung cancer management was published in 2010. These continuously updated documents aim to provide nationally uniform and evidence-based support in clinical decision-making [Citation5].
Start of the NLCR
The NLCR started to record data at a national level in 2002 with the primary aim of monitoring and improving quality of care and increasing knowledge on lung cancer [Citation9]. The register provides annual reports detailing clinical data and information on selected quality indicators, for example, decision-making in a multi-disciplinary conference (MDC) setting. In 2016, 77% of patients had been assessed in an MDC, an increase from 2014 (70%) [Citation4]. However, this varied substantially between hospitals, from 30% to 98%.
New diagnostic procedures and therapies
In the mid–1990s, positron emission tomography (PET) was introduced in Central Sweden and subsequently in other Swedish regions by use of mobile PET–scan units. In 2006, when information on the use of PET–scan was first recorded in the NLCR, 20% of patients initially considered for curative treatment (i.e., stages I–III) had undergone a PET in combination with computed tomography (PET–CT) to evaluate the presence of distant metastases [Citation4]. Today, almost 100% of these patients undergo PET–CT as part of their diagnostic workup. The use of EBUS started in the early 2000s for evaluation of the involvement of lymph nodes after a PET–CT, with increased use over time. Taken together, these new diagnostic procedures have led to more accurate staging and more correct treatment in relation to the true stage, likely contributing to the increase in the proportion of patients with stage IV disease and to the observed improvements in survival, especially in patients with stages I-III disease. Also, the use of stereotactic radiotherapy for patients with early-stage cancer considered inoperable, a treatment that began in the mid–1990s, has benefitted patients with early-stage lung cancer [Citation30,Citation31]. Our findings of improved survival in patients with stages I-III disease are in line with recent results from Estonia and Norway [Citation25–27].
Compared to patients with stage III disease, the observed improvements in survival were relatively modest in patients with stages I–II disease despite the increased use of PET–CT and EBUS. This finding may be explained by an already high survival in this patient group at the beginning of the study period (i.e., a ceiling effect) and that patients with stage III lung cancer have benefitted from new oncological treatment options.
Platinum-based chemotherapy was introduced in some regions in Sweden in the mid–1990s, and is now included in adjuvant treatment after surgery for patients with stages IB-III disease, in combination with radiotherapy for patients with stage III disease treated with curative intent who are not candidates for surgery, and as palliative treatment in patients with late-stage disease [Citation5].
The management of patients with targetable alterations (e.g., epidermal growth factor receptor [EGFR] mutations or anaplastic lymphoma kinase [ALK] rearrangement) has been affected by the introduction of targeted therapies from 2005 onwards, initially as later-line treatment and subsequently as first-line treatment. These molecular alterations are overrepresented in patients with adenocarcinoma and among never-smokers [Citation32–34], and may explain some of the improved outcomes in patients with adenocarcinoma and in never-smokers in the time period after the introduction. During the same time period, there was an indication of less pronounced improvements in survival for patients less likely to have a targetable alteration, for example, patients with squamous cell carcinoma and for ever-smokers. However, because of inconsistent results from previous studies, it remains unclear how the various subgroups have benefitted from the introduction of targeted therapies [Citation25–27]. This discrepancy may reflect differences between countries in both treatment guidelines and the pace of introduction of new targeted therapies.
Moreover, the introduction of targeted therapies and immunotherapies has recently provided new treatment options for patients with late-stage disease. Previously, improvements in the management of patients with stage IV disease have in general not been expected to affect five-year survival [Citation35]. However, this may be starting to change since we found some evidence of a more pronounced increase in relative survival in the time period after the introduction of targeted therapies and immunotherapies compared to before the introduction. Taken together, it will be of interest to monitor future survival patterns in patients with late-stage disease.
Considering the introduction and broader use of immunotherapy in recent years, it is reasonable to anticipate further improvements in survival for the overall lung cancer cohort, an improvement likely driven by patients with a late-stage disease without targetable alterations [Citation36,Citation37]. Previously these patients were treated with conventional platinum-based chemotherapy. However, with the introduction of immunotherapy, this patient group is also eligible for new treatment modalities.
Strengths and limitations
The main strength of our study was the population-based setting and the high completeness and quality of the data used, minimising the risk of selection and misclassification bias.
Assuming an increased survival over time, using the period approach would have underestimated the relative survival for the most recent years. We excluded patients with cancer diagnosed at autopsy, resulting in a slight overestimation of survival in all calendar years under study.
Because of a high smoking prevalence in patients with lung cancer, the risk of other comorbid conditions and the mortality experience from other causes is higher in patients with lung cancer compared to the general population. Hence, the main limitation of our study was the violation of the comparability assumption when estimating relative survival, i.e., the patient population was not comparable to a similar group in the general population with regard to sex and age [Citation38]. However, Hinchcliffe et al. assessed the influence of violating the comparability assumption and concluded that any bias introduced would be minor and will, if anything, result in an underestimation of the relative survival [Citation38].
Changes in the TNM–classification such as the reclassification in 2010 of pleural effusion from stage IIIB to stage IV could have contributed to the increase in patients with stage IV disease and to the improvements in survival in stage-specific subgroups [Citation4,Citation5,Citation39]. It appears that the improvement in relative survival between 2010 and 2011 was more pronounced in patients with stage III compared to those with stage IV disease.
The information on cancer treatment recorded in the NLCR is limited to planned primary treatment categorised as drug treatment, radiotherapy, surgery or other treatment. Thus, it was not possible to distinguish between patients who received immunotherapy, targeted therapy, or chemotherapy. Our findings are likely generalisable to settings with similar management of patients with lung cancer, and uptake of new diagnostic methods and treatments.
Conclusion
We found a substantial improvement in survival in the overall lung cancer population between 1995 and 2016. Of special interest was the indication of an improvement in the five-year survival in patients with stage IV disease. Our results corroborate previously observed global trends of improvements in the prognosis of patients with lung cancer. The reasons for the improved survival are multifactorial, including changes in and adherence to guidelines and uptake of new diagnostic procedures and therapies.
Author contributions
Conceptualisation, writing – review & editing, approval of the final manuscript: All authors. Data management: LL. Formal analysis: LL. Writing of original draft: LL. Funding acquisition: ML.
Supplemental Material
Download TIFF Image (23 MB)Supplemental Material
Download TIFF Image (15.3 MB)Supplemental Material
Download TIFF Image (23 MB)Supplemental Material
Download TIFF Image (15.3 MB)Supplemental Material
Download TIFF Image (7.7 MB)Supplemental Material
Download PDF (677.6 KB)Supplemental Material
Download MS Word (16.2 KB)Supplemental Material
Download MS Word (61.5 KB)Acknowledgement
This study was made possible by the continuous reporting by Swedish clinicians to the National Lung Cancer Register and the work done by the steering group of the register. We would like to thank Sarah Burkill for the language check.
Disclosure statement
Lukas Löfling, Shahram Bahmanyar, Helle Kieler, and Gunnar Wagenius have no conflict of interest to declare. Mats Lambe declares stock ownership in Pfizer and Astra Zeneca. The funding bodies had no role in the data collection and analysis and were not involved in the interpretation of results, writing, revision, or approval of the manuscript.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–241.
- Socialstyrelsen – Swedish National Board of Health and Welfare. Statistics on cancer incidence 2018. Stockholm, Sweden; 2019.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Regional Cancer Centre – Central Sweden. Lung cancer – National quality report, 2012-2016 Uppsala, Sweden; 2017. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/kvalitetsregister/rapport/nlcr_rapport_tom2016_171120_publicera.pdf
- Regionala Cancercentrum i Samverkan. Nationellt vårdprogram – Lungcancer [national care program – Lung cancer]. 4.0 ed. Uppsala, Sweden; 2020. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/nationellt-vardprogram-lungcancer.pdf
- Löfling L, Karimi A, Sandin F, et al. Clinical characteristics and survival in non-small cell lung cancer patients by smoking history: a population-based cohort study. Acta Oncol. 2019;58(11):1618–1627.
- Willén L, Berglund A, Bergström S, et al. Educational level and management and outcomes in non-small cell lung cancer. A nationwide population-based study. Lung Cancer. 2019;131:40–46.
- Löfling L, Bahmanyar S, Kieler H, et al. Antibiotic use prior to a lung cancer diagnosis: a population-based study. Cancer Causes Control. 2021;32(6):597–607.
- Regional Cancer Centre – Central Sweden. Swedish National Lung Cancer Register 2017 [170913]. Available from: http://www.cancercentrum.se/vast/cancerdiagnoser/lunga-och-lungsack/kvalitetsregister/
- Socialstyrelsen – Swedish National Board of Health and Welfare. The Swedish Cancer Register 2019 [updated 20190509;191122]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/swedish-cancer-register/
- Socialstyrelsen -Swedish National Board of Health and Welfare. The Swedish National Patient Register Socialstyrelsen -Swedish National Bord of Health and Welfare; 2019. [updated 20190520;191122]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/.
- Socialstyrelsen – Swedish National Board of Health and Welfare. The Swedish Prescribed Drug Register: Socialstyrelsen – Swedish National Board of Health and Welfare; 2019 [updated 20191018;191122]. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/lakemedelsregistret/.
- Socialstyrelsen – Swedish National Board of Health and Welfare. The Swedish Cause of Death Register: Socialstyrelsen -Swedish National Bord of Health and Welfare; 2019. [updated 20191018;191122]. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/dodsorsaksregistret/.
- Statistiska Centralbyrån (SCB) -Statistics Sweden. The Swedish Multi-generation Register 2016 [20171016]. Available from: https://www.scb.se/sv_/Vara-tjanster/Bestalla-mikrodata/Vilka-mikrodata-finns/Flergenerationsregistret/.
- Skatteverket – The Swedish Tax Agency. The Swedish Population Register 2020 200604. Available from: https://www.skatteverket.se/privat/folkbokforing/attvarafolkbokford/folkbokforingsdatabasen.4.3810a01c150939e893f16fe2.html.
- Statistiska Centralbyrån (SCB) -Statistics Sweden. Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) 2016 [20171026]. Available from: https://www.scb.se/contentassets/f0bc88c852364b6ea5c1654a0cc90234/dokumentation-av-lisa.pdf.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Regional Cancer Centre – Central Sweden. Swedish National Lung Cancer Register – Validation project. 2016.
- Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15(1):186–215.
- Human Mortality Database Team. Human Mortality Database [cited 2020 200430]. Available from: https://www.mortality.org
- Association of the Nordic Cancer Registers (NORDCAN). Glossary of statistical terms Lyon, France: International Agency for Research on Cancer (IARC); 2011. [cited 2019 20190716]. Available from: http://www-dep.iarc.fr/NORDCAN/english/glossary.htm
- R Development Core Team. R: a language and enviroment for statistical computing. 4.0.0. Vienna, Austria: R Foundation for statistical computing; 2020.
- StataCorp. Stata statistical software. 16.1. College station. Texas, United States of America: StataCorp; 2020.
- Lundberg FE, Andersson TML, Lambe M, et al. Trends in cancer survival in the Nordic countries 1990-2016: the NORDCAN survival studies. Acta Oncol. 2020;59(11):1266–1274.
- Brustugun OT, Grønberg BH, Fjellbirkeland L, et al. Substantial nation-wide improvement in lung cancer relative survival in Norway from 2000 to 2016. Lung Cancer. 2018;122:138–145.
- Innos K, Oselin K, Laisaar T, et al. Patterns of survival and surgical treatment in lung cancer patients in Estonia by histologic type and stage, 1996-2016. Acta Oncol. 2019;58(11):1–8.
- Nilssen Y, Strand TE, Fjellbirkeland L, et al. Lung cancer survival in Norway, 1997-2011: from nihilism to optimism. Eur Respir J. 2016;47(1):275–287.
- Brooks DR, Klint Å, Dickman PW, et al. Temporal trends in non-small cell lung cancer survival in Sweden. Br J Cancer. 2007;96(3):519–522.
- Statistiska Centralbyrån (SCB) -Statistics Sweden. Färre röker, fler snusar 2018 [20210222]. Available from: https://www.scb.se/hitta-statistik/artiklar/2018/farre-roker-fler-snusar/
- Nyman J, Hallqvist A, Lund JA, et al. SPACE – A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8.
- Haque W, Verma V, Polamraju P, et al. Stereotactic body radiation therapy versus conventionally fractionated radiation therapy for early stage non-small cell lung cancer. Radiotherapy and Oncology. 2018;129(2):264–269. Nov
- Donington JS, Colson YL. Sex and gender differences in non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2011;23(2):137–145.
- Isaksson S, Hazem B, Jönsson M, et al. Clinical utility of targeted sequencing in lung cancer: experience from an autonomous Swedish Health Care Center. JTO Clin Res Rep. 2020;1(1):100013.
- Du X, Shao Y, Qin H-F, et al. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer. 2018;9(4):423–430.
- Schabath MB, Thompson ZJ, Gray JE. Temporal trends in demographics and overall survival of non-small-cell lung cancer patients at Moffitt Cancer Center from 1986 to 2008. Cancer Control: Journal of the Moffitt Cancer Center. 2014;21(1):51–56.
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527.
- Regionala Cancercentrum i Samverkan. Nationellt vårdprogram – Lungcancer [National care program – Lung cancer] Uppsala, Sweden. 2021; 5.0. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/nationellt-vardprogram-lungcancer.pdf
- Hinchliffe SR, Rutherford MJ, Crowther MJ, et al. Should relative survival be used with lung cancer data?. Br J Cancer. 2012;106(11):1854–1859.
- American Joint Committee on Cancer. AJCC cancer staging manual. 7th edition. 7th ed. New York: Springer Verlag New York Inc; 2010.
