Abstract
Background
Immune checkpoint inhibitors (ICIs) are implemented as standard treatment for patients with advanced non-small cell lung cancer (NSCLC) in first-line and subsequent-line treatment. However, certain subgroups such as patients with older age, poor performance status (PS), and severe comorbidity are underrepresented in the randomized controlled trials (RCTs). This study aimed to assess overall survival (OS), treatment data, and clinical features affecting second- or subsequent-line ICI efficacy in an unselected, Danish, nationwide NSCLC population.
Methods
Patients with advanced NSCLC who started nivolumab or pembrolizumab as second-line or subsequent-line treatment between 1 September 2015, and 1 October 2018, were identified from institutional records of all Danish oncology departments. Clinical and treatment data were retrospectively collected. Descriptive statistics and survival analyses were performed.
Results
Data were available for 840 patients; 49% females. The median age was 68 years (19% were ≥75 years), 19% had PS ≥2, and 36% had moderate to severe comorbidity. The median OS (mOS) was 12.2 months; 15.1 months and 10.0 months in females and males, respectively. The median time-to-treatment discontinuation (mTTD) and median progression-free survival (mPFS) was 3.2 and 5.2 months, respectively. Patients with PS ≥2 had a mOS of 4.5 months, mTTD of 1.1 month, and mPFS of 2.0 months. In multivariable Cox regression analysis, male sex (HR = 1.35, 95% CI 1.11–1.62), PS >0 (PS 1, HR = 1.88, 95% CI 1.52–2.33; PS ≥2, HR = 4.15, 95% CI 3.13–5.5), liver metastases (HR = 1.72, 95% CI 1.34–2.22), and bone metastases (HR = 1.27, 95% CI 1.03–1.58) were significant poor prognostic OS factors.
Conclusions
Danish real-world patients with advanced NSCLC treated with second- or subsequent-line ICI had an OS comparable to results from RCTs. Women, frail and older patients constituted a higher proportion than in previous RCTs. Clinical features associated with poor OS were male sex, PS ≥1 (in particular PS ≥2), bone-, and liver metastases.
Background
Lung cancer is the leading cause of cancer-related mortality and morbidity worldwide, with a five-year survival rate ranging from 6% in advanced stages to 59% in early stages [Citation1]. In the Nordic countries, the lung cancer mortality has declined since the 1980s, due to improved diagnostics and treatment strategies [Citation2]. The latter include the implementation of immune checkpoint inhibitors (ICIs) as standard therapy, and despite the rapidly increasing use of first-line ICI as monotherapy or in combination with chemotherapy, some patients are ineligible for these regimens and may still be offered second-line ICI treatment [Citation3–11]. The pivotal randomized controlled trials (RCTs) had strict inclusion and exclusion criteria, not comparable to a real-world setting; thus, selecting patients for ICI treatment in a daily clinical setting remains challenging due to the lack of evidence in certain subgroups. These subgroups include patients with an old age, poor Eastern Cooperative Oncology Group (ECOG) performance status (PS), and severe comorbidity. Furthermore, the sex distribution in most international RCTs and real-world studies (RWS) is unequal, and thus less representative of the Nordic population, where NSCLC incidences are equal in men and women [Citation2,Citation12–14]. The median age of lung cancer patients in RCTs is 61 years; however, the median age in newly diagnosed Nordic patients with NSCLC is approximately 70 years [Citation3–5,Citation15,Citation16]. Thus, older patients and particularly patients aged ≥75 years, are greatly underrepresented in RCTs [Citation15,Citation17]. Lung cancer patients with PS ≥2 also constitute a substantial proportion of patients receiving oncologic treatment in the daily clinical setting [Citation18]. Nevertheless, frail patients with poor PS are typically underrepresented or not included in RCTs. Organ metastases are present in more than 50% of lung cancer patients at the time of diagnosis, and metastases to the brain, liver, and bone have been associated with impaired overall survival (OS) [Citation1,Citation19]. Moreover, comorbidity is frequent in lung cancer patients, and may affect their treatment and clinical outcome [Citation20–22]. However, neither level of comorbidity nor location of metastatic sites are reported in the RCTs [Citation3–5].
The primary aim of the present study was to report on OS in a Danish, comprehensive, consecutive population with advanced NSCLC, treated with ICIs in second-line or subsequent-line treatment. This implies a special attention to, and a comparison with RCTs of, the potential predictive or prognostic clinical features characterizing the subgroups of patients who are underrepresented in RCTs. These include those with higher age, poor PS, and more comorbidity. The secondary aims were to assess reasons for ICI discontinuation (including immune-related adverse events (irAEs)), treatment duration, and progression-free survival (PFS).
Methods
Study design and patients
A retrospective, nationwide real-world study (RWS) approved by the Danish Patient Safety Authority was conducted. Consecutive patients with NSCLC who received nivolumab or pembrolizumab in second-line or subsequent-line of palliative treatment between 1 September 2015, and 1 October 2018, were identified from institutional records. Data were collected from all (n = 11) Danish oncology departments.
Data collection and data management
Data were manually extracted from the electronic health record (EHR) systems. Clinical data were collected and stored in local databases at every oncology department. Covariates from the local databases were aligned according to variable names, values, and labels, and data were gathered into one dataset. Furthermore, data quality control was performed for each covariate. If the PS was described as a range, such as PS 1–2, in the EHR, the highest value was captured [Citation18]. Specific irAEs causing ICI discontinuation, and hospitalization and death due to irAEs were recorded. The disease stage and metastastic sites at ICI treatment initiation were retrospectively evaluated by reviewing baseline computed tomography (CT) scan reports.
Variables and endpoints
Baseline characteristics at ICI initiation included sex, age, PS, comorbidity according to Charlson Comorbidity Index Score (CCIS), smoking status, histopathological NSCLC subtype, TNM stage, metastatic locations, programmed death-ligand 1 (PD-L1) tumor proportion score (TPS), and epidermal growth factor receptor (EGFR) mutation status. When calculating the CCIS, the actual lung cancer diagnosis was excluded. Treatment data included the ICI drug, ICI start- and stop date, number of cycles administered (one cycle equals one administered dose), treatment line, and reasons for ICI discontinuation. These reasons were categorized as progressive disease (PD), poor PS, irAEs, and “other” reasons. Hospitalization and death due to irAEs were also recorded. The irAE types that were present at ICI discontinuation were recorded and classified as pneumonitis, hepatitis, skin toxicity, endocrinopathy, diarrhea/colitis, and ‘other toxicity’. Treatment could be discontinued for more than one reason, and more than one type of irAE could be present at treatment discontinuation. Patients received either nivolumab 3 mg/kg every two weeks, pembrolizumab 2 mg/kg every 3 weeks, or pembrolizumab 200 mg every three weeks. Individual dose intensities (mg/kg/time) were not recorded [Citation23]. The dates of progression and death were obtained from the EHRs. The progression date was defined as the date of the first clinical evidence of progressive disease (PD) (clinical examination leading to discontinuation of ICI) or radiological PD as verified by a CT and/or magnetic resonance imaging (MRI). The index date was the date of the first ICI administration, and the censoring date was 1 March 2020. The date of treatment discontinuation was the date of the last ICI administration. For living patients, the last follow-up date was defined as the date of the last patient contact in the EHRs. The primary aim was to asses OS, including investigation of predictive or prognostic clinical features. The secondary aims were to assess reasons for ICI discontinuation, treatment duration, and PFS.
Statistical methods
To compare baseline characteristics between sexes and PS groups, chi-square tests were used for the categorical variables, while the distributions of age were compared using Wilcoxon rank-sum test. No correction for multiple testing was performed. Kaplan–Meier (KM) estimates stratified by baseline variables and log-rank tests were used to assess OS, time to treatment discontinuation (TTD) and PFS. The median follow-up time was calculated using the reverse KM estimate. To adjust for multiple covariates and potential confounders, a multivariable Cox regression analysis was performed. Initially, the assumption of proportional hazard functions was assessed for each of the baseline categorical variables by visual inspection of the log-minus-log survival curves and formally tested using the Grambsch-Therneau proportional hazard test with survival times transformed by the KM estimate. PS, bone-, liver-, adrenal- and distant lymph node metastases, histopathology, and EGFR mutation status violated the proportional hazards assumption. Therefore, average hazard ratios were estimated by weighted Cox regression [Citation24]. Weighted univariable and multivariable Cox regression models were used for analysis of the association between OS and all the baseline categorical variables (except for TNM stage). Comorbidities that were present in >5% of the cases, were included in the weighted univariable Cox regression analysis. For the KM estimate and Cox regressions, CCIS was categorized as CCIS 0–1 and CCIS ≥2 [Citation25].
A p-value of 0.05 was defined as the threshold of statistical significance. All analyses were performed using R version 4.0.2 (R Core Team, Vienna, Austria) [Citation26]. The survival package was used to assess the assumption of proportional hazard functions, the ggsurvplot package for visualizing KM estimates, and the coxphw package for the weighted Cox regression analyses.
Results
Baseline characteristics
We identified 841 consecutive patients. No patients were lost to follow up. A single patient harboring an ALK translocation was excluded, leaving 840 patients with a median follow-up time of 34.7 months (95% confidence interval (CI) 33.2–35.9) eligible for analysis.
The median age was 68 years, with 19% ≥75 years, and 5% ≥80 years. A total of 19% of the patients (n = 158) had PS ≥2, 57% (n = 479) had PS 1, and 22% (n = 182) had PS 0. PS was missing in 2% of the patients (n = 21). Distant metastases were present in 86% of the patients. CCIS ≥2 was observed in 36% (n = 301) of the patients. The prevalence of specific comorbidities according to CCIS is summarized in Supplementary Table 1. The baseline characteristics of the patients are summarized in .
Table 1. Baseline characteristics.
Male patients had a higher age (p = 0.001) and more comorbidities (p < 0.0001) than females. Squamous cell carcinomas were more frequent among male (49%) than female patients (23%) (p < 0.0001). Brain metastases were more prevalent in women than in men (p < 0.0001) (Supplementary Table 2).
Patients with baseline PS ≥2, compared to PS 0–1, consisted of more male patients (58%, p = 0.046), and received fewer nivolumab/pembrolizumab cycles (2/3 vs. 7/8) (Supplementary Table 2).
ICI Treatment
At the censoring date, 99% (n = 831) had discontinued ICI. ICI treatment characteristics are demonstrated in .
Table 2. ICI treatment characteristics.
The median TTD (mTTD) was 3.2 (95% CI 2.8–3.6) months. In patients with PS ≥2, the mTTD was 1.1 (95% CI 0.7–1.4) month compared to 3.3 (95% CI 2.8–3.8) and 6.0 (95% CI 5.1–7.8) months in PS 1 and PS 0 patients, respectively.
Clinical outcomes
The mOS was 12.2 (95% CI 10.8–13.8) months, and the 1- and 2-year OS rates were 50% (95% CI 47–54) and 30% (95% CI 27–33), respectively (). The estimated three-year OS rate was 20% (95% CI 17–23). The mOS was 15.1 and 10.0 months in female and male patients, respectively. The mOS for patients with PS ≥2 was 4.5 months compared to 12.2 and 22.1 months in patients with PS 1 and PS 0, respectively (). The mPFS was 5.2 (95% CI 4.5–6.9) months (), and 2.0 months in patients with PS ≥2.
Table 3. Overall and progression-free survival according to sex and performance status.
Prognostic clinical features
Kaplan-Meier estimates demonstrated that OS was reduced in men (p < 0.0001), in patients with PS >0 (p < 0.0001), and in patients with bone (p = 0.003) and/or liver metastases (p < 0.0001) ().
Figure 1. OS stratified by ECOG PS, sex and histopathology, liver metastases and bone metastases. OS: overall survival; ECOG PS: Eastern Cooperative Oncology Group performance status; F: female; M: male; A: adenocarcinoma; S: squamous cell carcinoma.
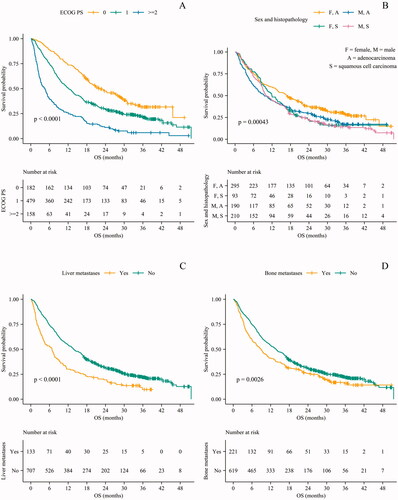
Age ≥75 years, comorbidity according to CCIS, and the presence of brain metastases at ICI initiation were not significantly associated with impaired OS (Supplementary Table 3).
In multivariable Cox regression analysis, male sex (HR = 1.35; 95% CI 1.11–1.62), liver metastases (HR = 1.72; 95% CI 1.34–2.22), and bone metastases (HR = 1.27; 95% CI 1.03–1.58) remained statistically significant poor prognostic factors. Likewise did PS ≥2 (HR = 4.15; 95% CI 3.13–5.50) and PS 1 (HR = 1.88; 95% CI 1.52–2.33) compared to PS 0. Age ≥75 years (HR = 0.99; 95% CI 0.8–1.23), and the presence of brain metastases at ICI initiation (HR = 1.1; 95% CI 0.82–1.47) did not significantly affect OS (). EGFR mutation status and PD-L1 TPS were unknown in 33% and 29% of cases, respectively. PD-L1 ≥ 50% was associated with an improved OS (HR = 0.69; 95% CI 0.48–0.98).
Figure 2. Weighted multivariable Cox regression analysis, with forest plots showing average hazard ratios (HR). CI: confidence interval; ECOG PS: Eastern Cooperative Oncology Group performance status; CCIS: Charlson Comorbidity Index Score; NSCLC: non-small cell lung cancer; EGFR: epidermal growth factor receptor; PD-L1: programmed death-ligand 1
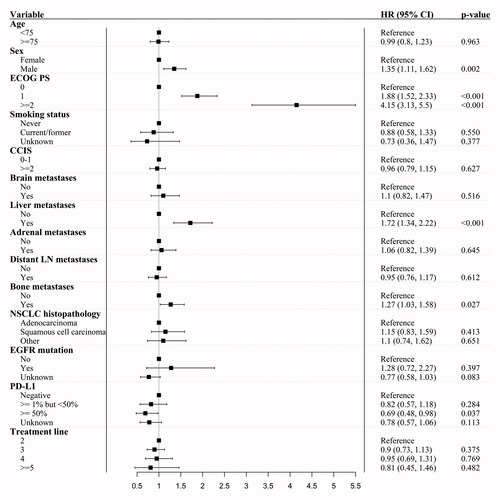
Extension of the multivariable Cox regression with interaction between sex and histopathology demonstrated a significantly poorer OS in patients with adenocarcinoma, if they were male rather than female, while no difference in OS were seen between sexes for patients with squamous cell carcinoma (Supplementary Table 5).
Kaplan-Meier estimates demonstrated that factors associated with a poor PFS were male sex (p = 0.006), ECOG PS >0 (p < 0.0001), no history of smoking (p = 0.03), liver metastases (p < 0.0001), a positive EGFR mutation status (p = 0.004), and PD-L1 < 1% (p < 0.0001) (Supplementary Table 4).
Discussion
Several subgroups have been underrepresented in RCTs, and therefore, focus is increasingly placed on the importance of gathering clinically relevant data from RWS, which typically represent a more unselected treatment population. However, different global health care systems affect the populations included in RWS. In Denmark, according to the Danish Health Care Act, all patients are offered treatment according to national treatment guidelines, irrespective of their income, education, and residential and socioeconomic status, which minimizes the risk of selection bias in Danish studies [Citation27]. Treatment with ICIs is expensive and holds a potential risk of causing severe irAEs. Thus, characterizing a large cohort of real-life patients in detail may contribute with important knowledge helping clinicians make more evidence-based decisions on whether to offer patients ICI or not.
In this large nationwide NSCLC study of real-world ICI efficacy, the mOS and the 1-year OS rate were comparable to results from previous anti-PD-1 clinical trials of pretreated patients [Citation3–5,Citation28,Citation29]. An improved mPFS compared to results from the RCTs, could be explained by differences in PFS definition [Citation3–5].
Lung cancer incidence and mortality remain higher in males than females in some countries [Citation30,Citation31]. However, in agreement with the narrowing gap in the lung cancer incidence between sexes in Nordic countries, half of the patients in our study were females, as opposed to a lower proportion reported in comparable RCTs and RWS [Citation2,Citation13,Citation14]. In RCTs, ICI significantly improved OS in both men and women compared to chemotherapy, however, the benefit seemed to be higher in men [Citation7,Citation13]. In this study, PS ≥2, higher CCIS, and squamous cell carcinomas were more frequent in males as compared to females. Despite adjusting for these factors, male patients with adenocarcinomas had a worse OS than female patients with adenocarcinomas.
In our study, the median age was 68 years, which is 5–7 years older than patients included in the anti-PD-1 RCTs, and more comparable to the age of real-world lung cancer patients [Citation3–5,Citation15,Citation16]. Especially data on patients aged ≥75 years is lacking in RCTs. However, in our study they constituted 19% of patients, compared to only 7%–8% in previous RCTs [Citation3,Citation4]. Even with this greater proportion of older patients, the mOS was comparable to results from previous clinical trials and RWS, as age did not significantly affect OS [Citation3,Citation4,Citation28,Citation29,Citation32,Citation33]. Our data demonstrate that ICI should not be excluded as a treatment option because of high chronological age.
As opposed to the RCTs, the proportion of PS ≥2 patients in our study (19%) reflects the overall fraction of patients with NSCLC and PS ≥2 [Citation18]. Thus, compared to the RCTs, our study included more frail and heavily pretreated patients, with more than one third receiving third-line or further subsequent-line ICI treatment [Citation3–5]. Nevertheless, the mOS of patients with PS ≥2 was comparable to results from clinical trials, pooled analyses and other RWS [Citation28,Citation29,Citation34]. In contrast to this, the PePS2 study assessed the efficacy of pembrolizumab in 60 patients with PS ≥2, and reported a mOS of 12.1 months in previously treated patients [Citation35]. However, since the mPFS was only 2.0 months and the mTTD was only 1.1 month in our study, the clinical benefit of ICIs is very limited in most of these patients. On the other hand, we report a mOS of 22.1 months in patients with PS 0, which is comparable to the mOS of PS 0–1 patients treated with first-line ICI in RCTs [Citation6,Citation36]. This illustrates that PS 0 patients may benefit particularly from ICIs, even when administered in subsequent lines. However, the mOS of PS 0 and PS 1 patients, has not been compared in RCTs and rarely in RWS of second-line ICI [Citation3–5,Citation37,Citation38].
A large proportion of the patients in our study had metastatic disease (86%), which is representative of the palliative NSCLC population. However, in most RCTs, information regarding metastatic sites is rarely available, despite the known prognostic impact [Citation3–5,Citation28,Citation29]. In the present study, bone- and liver metastases were significant poor prognostic factors for OS, whereas brain metastases did not affect OS. This is comparable to results from other RWS [Citation19,Citation39–42]. In most patients, brain metastases are stable at ICI initiation due to previous local therapy with radiotherapy or neurosurgery. In our study, not all patients had a MRI of the brain prior to ICI initiation, thus the actual number of patients with brain metastases, as opposed to those with liver metastases, were not known at baseline. These factors may explain the lack of impact on OS of brain metastases. Poor PS, liver and bone metastases er known poor prognostic factors, and based on our results, it is difficult to assess whether these patients actually could benefit from ICI compared to best supportive care or subsequent line chemotherapy. However, our results imply that careful consideration should be made before administering ICI to particularly patients with PS ≥2.
In accordance with another RWS, no association between comorbidity and OS was observed [Citation42]. However, comorbidities are rarely reported in RWS of ICI-treated patients with advanced NSCLC.
Strengths and limitations
The strengths of this study are the inclusion of a nationwide unselected population of all Danish patients with NSCLC treated with ICI in second-line or further subsequent line, the completeness of follow-up for all patients, and the large sample size, allowing for strong subgroup analyses. The study had some limitations. The retrospective nature of the study, reduced the validity of the comorbidity data, which preferably should be prospectively collected. Likewise for smoking status, ECOG PS, grade of toxicity by the Common Toxicity Criteria (CTC), and tumor response evaluation according to Response Evaluation Criteria in Solid Tumors [Citation43,Citation44]. Laboratory data and data regarding potential confounders such as prior or concomitant glucocorticoid and antibiotic administration and body mass index were also not obtained [Citation44–46].
Conclusion
The OS of ICI-treated patients in our study, was comparable to the OS demonstrated in RCTs [Citation3–5]. Women accounted for half of the patients in this Danish cohort, making the results from this cohort especially comparable to other countries (including Nordic countries) with a high proportion of female NSCLC patients eligible for ICI. Furthermore, our results showed that older age did not affect ICI efficacy, and ICIs should not be excluded as a treatment option, due to high chronological age. Patients with PS ≥2 had only very limited effect of ICI with a very poor prognosis, thus careful consideration should be made on an individual basis when offering ICIs to this subgroup. Data on metastatic sites should be available in future RCTs, because of the prognostic impact on OS and in order to improve the comparison between future RCTs and RWS.
Ethics approval and consent to participate
Approved by the Danish Patient Safety Authority and reported to The Danish Data Protection Agency.
Consent for publication
Patient consent was waived by the Danish Patient Safety Authority, due to the retrospective design of the study, and the use of routinely collected data.
Supplemental Material
Download MS Word (43.1 KB)Acknowledgements
The authors thank Professor Ursula G. Falkmer, MD, PhD, Clinical Cancer Research Center, Departments of Clinical Medicine and Oncology, Aalborg University and Aalborg University Hospital for contributing to the writing process.
Disclosure statement
The funding sources were not involved in the study design, collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. GF Persson: Advisory board Roche, Astra Zeneca, BMS, MSD, Takeda, Pfizer. Congress travels with Roche, Astra Zeneca, BMS, MSD, Takeda, Pierre Fabre. Research grants from Varian Medical Systems. M Pøhl: Honoraria for lectures and consultancy from AstraZeneca, BMS, MSD, Pfizer, Roche. SW Langer: Advisory board MSD, Roche, Pfizer. The remaining authors declare no conflict of interest.
Data availability statement
The study data may be available on request from the corresponding author, Mette T Mouritzen. The data are not publicly available due to the General Data Protection Regulation.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
- NORDCAN. March 26, 2019. https://www-dep.iarc.fr/nordcan/dk/StatsFact.asp?cancer=180&country=0. [accessed September 2021].
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092.
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051.
- Medicinrådet. Pembrolizumab (Keytruda) i kombination med kemoterapi (genvurdering). October 21, 2020. https://medicinraadet.dk/anbefalinger-og-vejledninger/laegemidler-og-indikationsudvidelser/m-p/pembrolizumab-keytruda-ikke-planocellulaer-ikke-smacellet-lungekraeft-genvurdering. [accessed September 2021]
- Medicinrådet. Pembrolizumab (Keytruda) i kombination med platinbaseret kemoterapi (genvurdering). January 27, 2021. https://medicinraadet.dk/media/hbqdv2rt/medicinr%C3%A5dets_anbefaling_vedr-_pembrolizumab_i_komb-_m-_kemoterapi_til_nsclc-vers-_1-0_adlegacy.pdf. [accessed September 2021]
- DOLG. DOLG referenceprogram. 2018. http://dolg.dk/referenceprogram/referenceprogram-kapitel-7/. [accessed September 2021]
- La J, Cheng D, Brophy MT, et al. Real-world outcomes for patients treated with immune checkpoint inhibitors in the veterans affairs system. JCO Clin Cancer Inform. 2020;4:918–928.
- Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746.
- Conforti F, Pala L, Bagnardi V, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781.
- Sacher AG, Le LW, Leighl NB, et al. Elderly patients with advanced NSCLC in phase III clinical trials: Are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol. 2013;8(3):366–368.
- Ekman S, Horvat P, Rosenlund M, et al. Epidemiology and survival outcomes for patients with NSCLC in scandinavia in the preimmunotherapy era: a SCAN-LEAF retrospective analysis from the I-O optimise initiative. JTO Clin Res Rep. 2021;2(5):100165
- Landre T, Des Guetz G, Chouahnia K, et al. Immune checkpoint inhibitors for patients aged ≥ 75 years with advanced cancer in first- and second-line settings: a Meta-analysis. Drugs Aging. 2020;37(10):747–754.
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: Implications for research. J Thorac Oncol. 2008;3(2):125–129.
- Campos-Balea B, de Castro Carpeño J, Massutí B, et al. Prognostic factors for survival in patients with metastatic lung adenocarcinoma: an analysis of the SEER database. Thorac Cancer. 2020;11(11):3357–3364.
- Gouliaev A, Hilberg O, Christensen NL, et al. Comorbidity among danish lung cancer patients before and after initial cancer diagnosis. Eur Clin Respir J. 2020;8:1861579.
- von Itzstein MS, Gonugunta AS, Mayo HG, et al. Immunotherapy use in patients with lung cancer and comorbidities. Cancer J. 2020;26(6):525–536.
- Sculier JP, Botta I, Bucalau AM, et al. Medical anticancer treatment of lung cancer associated with comorbidities: a review. Lung Cancer. 2015;87(3):241–248.
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43.
- Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted cox regression. Statist Med. 2009;28(19):2473–2489.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Team RC. R: A language and environment for statistical computing. https://www.R-project.org/. [accessed September 2021]
- Danish Lung Cancer Group. National Clinical Guidelines for the treatment of Lung cancer. https://www.lungecancer.dk/referenceprogram/. [accessed September 2021.
- Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: a phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–172.
- Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J Thorac Oncol. 2019;14(9):1628–1639.
- Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1629–1637.
- Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174–1182.
- Luciani A, Marra A, Toschi L, et al. Efficacy and safety of anti-PD-1 immunotherapy in patients aged≥ 75 years with non-small-cell lung cancer (NSCLC): an Italian, multicenter, retrospective study. Clin Lung Cancer. 2020;21(6):e567–e571.
- Elkrief A, Richard C, Malo J, et al. Efficacy of immune checkpoint inhibitors in older patients with non-small cell lung cancer: Real-world data from multicentric cohorts in Canada and France. J Geriatr Oncol. 2020;11(5):802–806.
- Dall'Olio FG, Maggio I, Massucci M, et al. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. 2020;145:95–104.
- Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med. 2020;8(9):895–904.
- Mok TSK, Wu YL, Kudaba I, KEYNOTE-042 Investigators, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830.
- Passaro A, Attili I, Morganti S, et al. Clinical features affecting survival in metastatic NSCLC treated with immunotherapy: a critical review of published data. Cancer Treat Rev. 2020;89:102085.
- Mencoboni M, Ceppi M, Bruzzone M, et al. Effectiveness and safety of immune checkpoint inhibitors for patients with advanced non small-cell lung cancer in real-world: review and meta-analysis. Cancers (Basel). 2021;13(6):1388.
- Yang K, Li J, Bai C, et al. Efficacy of immune checkpoint inhibitors in non-small-cell lung cancer patients with different metastatic sites: a systematic review and meta-analysis. Front Oncol. 2020;10:1098.
- Botticelli A, Cirillo A, Scagnoli S, et al. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines (Basel). 2020;8(2):203.
- Landi L, D'Incà F, Gelibter A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7(1):316.
- Bjørnhart B, Hansen KH, Jørgensen TL, et al. Efficacy and safety of immune checkpoint inhibitors in a danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol. 2019;58(7):953–961.
- Hsiehchen D, Watters MK, Lu R, et al. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open. 2019;2(9):e1911519
- De Giglio A, Mezquita L, Auclin E, et al. Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers (Basel). 2020;12(10):2827.
- Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444.
- Cortellini A, Ricciuti B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403.
