Abstract
Background
Data on the age-specific incidence of esophageal cancer are lacking. Our aim was to investigate the age-stratified incidence, treatment, and survival trends of esophageal cancer in the Netherlands, with a focus on adults <50 years.
Material and methods
Patients diagnosed with esophageal cancer were included from the nationwide Netherlands Cancer Registry (1989–2018). Follow-up data were available until 31 December 2018. Annual percentage changes of incidence were analyzed according to age group (<50, 50–74, and ≥75 years) and histology type: adenocarcinoma (EAC) and squamous cell carcinoma (ESCC). Treatment trends and relative survival rates (RSR) were estimated by age and stage grouping.
Results
A total 59,584 patients were included. In adults <50 years, EAC incidence tripled (mean increase per year: males 1.5%, females 3%), while the incidence of ESCC decreased (mean decrease per year: males −5.3%, females −4.3%). Patients <50 years more often presented with advanced disease stages compared to older patients and were more likely to receive multimodality treatments. Most patients <50 years with potentially curable disease were treated with neoadjuvant chemoradiotherapy followed by surgery compared to patients 50–74 and ≥75 years (74% vs. 55% vs. 15%, respectively; p < .001), and received more frequent systemic therapy once staged with palliative disease (72% vs. 54% vs. 19%, respectively; p < .001). The largest RSR improvement was seen in patients <50 years with early-stage (five years: +47%), potentially curable (five years: +22%), and palliative disease (one year: +11%). Over time, a trend of increasing survival difference was seen between patients <50 and ≥75 years with potentially curable (five-year difference: 17% to 27%) and palliative disease (one-year difference: 11% to 20%).
Conclusion
The incidence of EAC is increasing in adults <50 years in the Netherlands. Differences in the use of multimodality treatments with curative or life-prolonging intent in different age categories may account for increasing survival gaps.
Background
According to recent global cancer burden estimates, esophageal cancer (EC) is worldwide the seventh most common malignancy in terms of incidence (2020: 604,000 new cases) with an increase of more than 50% in the number of new cases over the past three decades [Citation1,Citation2]. The Netherlands has seen one of the highest EC incidences in Europe with 15.8 new cases per 100,000 in 2020, and these numbers are expected to rise until 2040 [Citation3,Citation4]. Furthermore, survival of Dutch patients diagnosed with potentially curable EC has improved remarkably over the last 15 years [Citation5]. This can mainly be attributed to the introduction of multimodality treatment with neoadjuvant chemoradiotherapy combined with surgery, nationwide centralisation of surgical treatment and improved staging procedures [Citation6–8].
However, some concerns exist that not all EC patients benefit equally from these improvements, comparable to trends seen in gastric cancer [Citation9,Citation10]. Studies concluded that patients <70 years with potentially curable gastric cancer have a stronger survival improvement compared with older patients due to higher rates of surgery and perioperative treatment compliance [Citation9,Citation11]. Similarly, young patients with potentially curable EC were more likely to undergo surgery and systemic treatment than older patients [Citation12].
The epidemiology of gastrointestinal malignancies is currently changing. It is well-known that the incidence of esophageal squamous cell carcinoma (ESCC) has substantially decreased in high-income countries [Citation13]. Other studies have shown that the incidence of colorectal cancer has started to increase in patients <50 years [Citation14]. Recent population-based evidence from the USA suggested an increasing incidence of esophageal adenocarcinoma (EAC) in patients <50 years since 1997 [Citation15].
Age-specific incidence of EC in Europe has not yet been investigated on a population-based level. The aim of this study was therefore to estimate trends in EC incidence in the Netherlands in different age groups, focusing on relatively young adults and to describe stage-specific treatment and survival trends in young patients compared with older patients.
Material and methods
Study design and data collection
Data were obtained from the nationwide Netherlands Cancer Registry including all newly diagnosed malignancies since 1989, with 31 December 2018 as last day of follow-up. The International Classification of Diseases for Oncology (ICD-O) codes were used to classify tumor morphology and topography. Tumor stage was recorded according to the TNM-classification of the Union for International Cancer Control that was in use in the year of diagnosis. Tumors of the lower thoracic esophagus with celiac lymph node metastasis classified as M1a according to TNM-5/6 were reclassified to M0 based on TNM-7. Follow‐up of all patients was complete up to January 2020 (pre-COVID-19) through annual linkage with the Municipal Personal Records Database. The reporting of this study is according to the STROBE guidelines.
Study population
Patients with primary EAC, ESCC or carcinoma ‘not otherwise specified’ of the esophagus or esophagogastric cardia (C15.0–C16.0) diagnosed in the period 1989–2018, were included. Other histology types, such as stromal, neuro-endocrine or adenosquamous tumors, were excluded (N = 2039).
Definitions
Patients were stratified into three age groups: <50, 50–74, and ≥75 years. Treatment stage grouping was based on cTNM-stage: early-stage (cT1N0M0/X), potentially curable (cT1N+/X and cT2-4a with any cN and cM0/X), and palliative stage EC (cT4b and/or cM1). In this study, cN stage was classified in N0 or N+. The study period was categorised based on important developments in EC care: 1989–2003 (before centralisation of surgery), 2004–2008 (centralisation of surgery), 2009–2013 (introduction of neoadjuvant chemoradiotherapy and endoscopic resection), and 2014–2018 (neoadjuvant CROSS-chemoradiotherapy considered standard of care, further centralisation and introduction of upper-GI-specific multidisciplinary tumor boards).
Treatment modalities
Treatment was defined according to tumor stage, with esophagectomy, neoadjuvant chemo(radio)therapy with surgery, and chemo(radio)therapy without surgery as treatment modalities. In early-stage EC endoscopic resection was also included as primary treatment. Palliative stage EC additionally included systemic therapy alone, external beam radiotherapy, and/or brachytherapy. The ‘chemo(radio)therapy without surgery’ includes both palliative patients with concurrent chemoradiotherapy as patients with sequential chemotherapy and radiotherapy. Patients not receiving any of the defined treatments were categorised to ‘no/other treatment’ group.
Statistical analysis
Crude rate (CR) and the European Standardised Rate (ESR; Eurostat v.2013) incidence rates for the age groups were calculated as the number of new cases per 100,000 person-years. Incidence trends were estimated by Joinpoint regression analyses, using the Joinpoint Regression Programme 4.8.0.1 (National Cancer Institute; USA). The estimated annual percentage change (EAPC) was calculated to quantify the percentual change in EC incidence per year. For more detailed assessment of incidence, patients were stratified into smaller age groups: 0–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years. The relative survival rate (RSR) was used as proxy for disease-specific survival and can be interpreted as the ratio of the observed survival in cancer patients to the expected survival in a comparable cohort from the general population adjusted for age, sex, and calendar year. For the estimation of the expected survival lifetables of the Dutch general population were obtained from Statistics Netherlands (CBS), which included sex-specific annual survival probability data for persons aged 0–99 years between 1989 and 2018. Five-year and one-year relative survival rates (RSRs) were estimated for different time periods stratified by age group and tumor stage using the Pohar Perme method. p < .05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics v25.0 (IBM Corp., Armonk, NY), SAS/STAT® v9.4 (SAS Institute, Cary, NC), STATA v13.0 (Statcorp LP, College Station, TX).
Results
Baseline characteristics
A total of 59,584 patients was included in the analyses. Baseline patient and tumor characteristics are shown in . Six percent of patients were <50 years, 63% were 50-74 years and 31% were ≥75 years. The percentage of patients <50 years decreased from 7.9% to 3.8% due to a proportionally larger increase of patients 50–74 years (Supplementary Table 2). In patients <50 years, advanced cT-stage (cT4a/b: 13%), positive regional lymph nodes (cN+: 53%), and distant metastases (cM1: 39%) were more frequently seen when compared to older patients. Patients <50 years had the highest percentage of palliative disease at the time of diagnosis (47%, p < .001) compared to patients ≥75 years. The proportion of patients registered with cTx and/or cNx have been analyzed and presented in Supplementary table 1. These registries decreased significantly over time for all stages and all age categories in a similar rate. The cTxNxM0 cases were all considered as ‘potentially curable’ stage because they were staged as cM0. It is unlikely that these cases were early-stage tumors and treated as such, because the cT- and cN-stage would then probably have been known (Supplementary Table 1B).
Table 1. Baseline characteristics of study population according to pathological tumor response.
Trends in incidence
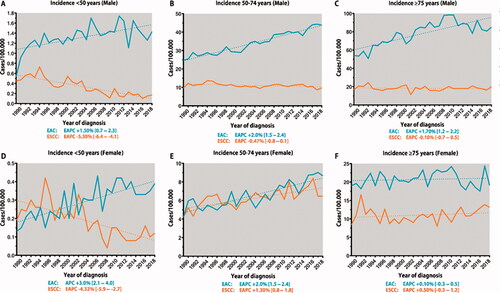
Sensitivity analysis showed minimal differences between the age-truncated CR and ESR estimates; therefore, only the CR incidence is presented. Adults <50 years showed similar incidence trends in both sexes. Overall, incidence of EAC in adults <50 years tripled from 0.34 per 100,000 in 1989 to 0.92 per 100,000 in 2018 (EAPC 1.7%, CI 1.0–2.4). This increase was particularly seen in females with an EAPC of 3.0% (CI 2.1–4.0) compared to 1.5% (CI 0.7–2.3) in males (). The incidence of ESCC showed a persistent decrease in males and females <50 years with an EAPC of –5.3% (CI –6.4 to −4.1) and –4.3% (CI –5.9 to –2.7), respectively. A significant annual incidence increase of EAC was also seen in adults aged 50–74 years (). In females aged 50–74 years, there was a notable increase in the incidence of ESCC (EAPC 1.3%, CI 0.8–1.8; ). In the age group ≥75 years, the incidence of EAC was only rising in males (EAPC 1.7%, CI 1.2–2.2; ), with stable annual ESCC incidence rates in males and females (). Further stratification of incidence rates to smaller age groups showed that the rising incidence of EAC already starts from the age of 40 years in both males and females (Supplementary Figure 1(A,C)). The increasing incidence of ESCC in females occurred from the sixth decade onwards (Supplementary Figure 1(B,D)).
Trends in treatment modalities
Treatment trends by age category and tumor stage are shown in . No major treatment differences were seen between age groups with early-stage EC. Endoscopic resection, introduced in 2001–2002 in the Netherlands, almost completely replaced esophagectomy as treatment of choice for early-stage cancer (). The impact of endoscopic resection was most prominent in patients ≥75 years, with 81% receiving curative treatment in 2018.
Figure 2. Treatment trends in age- and stage-specific esophageal cancer (EC) in the Netherlands, period 1989–2018. nCRT: neoadjuvant chemoradiotherapy; nCT: neoadjuvant chemotherapy.
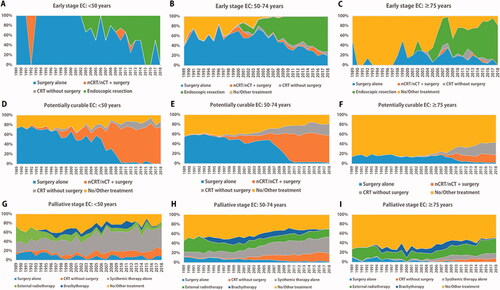
Upfront esophagectomy for patients diagnosed with potentially curable EC has gradually been replaced since 2007 by multimodality treatment, i.e. neoadjuvant chemoradiotherapy combined with surgery (). This trend was seen in all age groups. However, this surgical practice was relatively stable over the years. The percentage of patients receiving chemoradiotherapy alone increased over time in patients 50–74 and ≥75 years. The percentage of patients ≥75 years receiving no/other treatment was quite high compared to younger patients (). Yet, there was a notable increase in the use of chemoradiotherapy alone in patients ≥75 years (ESCC +28%, EAC +9).
A wide variation of treatment modalities was seen in palliative disease. Overall, tumor-directed treatment increased, +50% in patients <50 years, +53% in patients 50–74 years, and +25% in patients ≥75 years. Surgery in the palliative stage remained rare, almost only performed in a minority of younger patients (8%). Use of systemic therapy alone or chemoradiotherapy increased by 40% in patients <50 and 50-74 years, and by 18% in patients ≥75 years. The use of external beam radiotherapy increased most notably in the oldest age group (+15%). Since its introduction in 1991, increasing numbers of patients received brachytherapy, but this trend has strongly declined in recent years.
Trends in survival
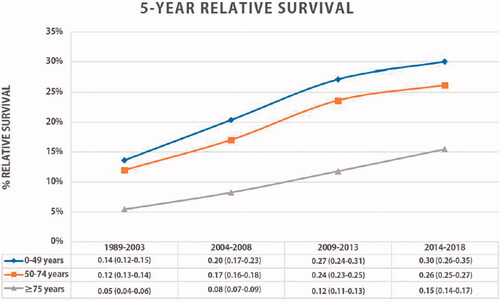
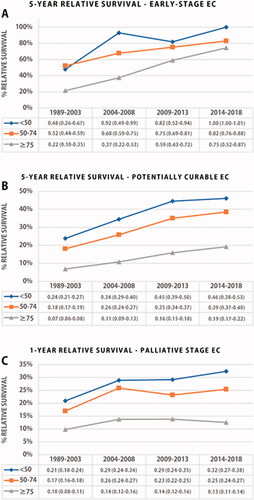
Overall, the five-year RSR of EC patients improved significantly over time in all age categories (). In general, patients <50 years gained more survival benefit over time compared to patients aged 50–74 and ≥75 years (+16% vs. +14% vs. 10%). The five-year RSR difference between patients <50 years and ≥75 years was 15% in recent time periods. The largest stage-based RSR improvement was seen in patients <50 years diagnosed with early-stage EC (), with a significant five-year RSR improvement to 100% (+52%). The survival difference between the youngest and the oldest age group with early-stage disease gradually decreased over the years from 55% in 2004–2008 to 26% in 2009–2018. Young patients showed the greatest improvement in five-year RSR in potentially curable EC (+22%), and one-year RSR in palliative EC (+11%). The survival gap between the <50 and ≥75 years group increased over time in potentially curable and palliative staged disease, from 17% to 27% and 11% to 20%, respectively.
Discussion
This population-based study including data from the past three decades in the Netherlands showed an increasing incidence of EAC in adults <50 years. The annual increase in younger females was larger than in younger males (3% vs. 1.5%). In contrast, the incidence of ESCC decreased most rapidly in this age group.
Epidemiological studies have provided important information on the incidence and future burden of EC according to histology type, sex, and ethnicity [Citation15,Citation16]. However, little attention has been given to age-specific incidence rates. One recent population-based study from the USA reported an increased EAC incidence in females and non-Hispanic white males <50 years [Citation15]. We found that the incidence of EAC increased more rapidly in females <50 years. This finding is in line with the accelerating cancer incidence rates in younger females [Citation17]. Because of small absolute numbers, changing EC incidence trends in younger adults may not have been observed in small sample size single-center studies [Citation18,Citation19].
As with other gastro-intestinal cancers, the emerging trend of EAC in young adults may be explained by the growing unhealthy dietary and lifestyle patterns associated with central obesity, reduced physical activity, and other metabolic changes [Citation20]. In fact, excessive body weight and associated gastroesophageal reflux disease are major risk factors for the development of Barrett’s esophagus, a well-known precursor of EAC [Citation20]. Other factors such as smoking and alcohol consumption, commonly associated with ESCC, are additional contributors to the development of EAC [Citation13,Citation20]. Interestingly, the incidence of ESCC was found to have declined in adults <50 years, but rather increased in females 50–74 years. This latter trend may be explained historically by the high smoking prevalence in females in the Netherlands since the 1960s before starting to drop in the late 1970s, while showing a steady decrease in males from the early 1960s [Citation21,Citation22]. Delayed smoking cessation patterns have recently also been associated with an increased incidence of lung cancer in females aged 50–54 years compared to a declining trend in males [Citation23,Citation24].
Our findings emphasise the need to reverse this alarming trend. Preventive strategies including community- and clinician-based awareness programs focusing on early symptom recognition and healthy life-styles are needed but may not be sufficient. Strategies to reduce future burden should include early detection of EC in at-risk patients, such as those with Barrett’s esophagus, as early-stage EC is associated with an excellent prognosis [Citation25,Citation26]. Since the great majority of EACs are diagnosed outside Barrett’s esophagus surveillance programs [Citation27], further investigations should focus on the development of (cost)effective screening strategies. Promising developments in the field of minimally invasive screening techniques include the use of breath analysis with portable electronic nose devices [Citation27] and the minimally invasive collection of esophageal cells with ingestible devices [Citation28,Citation29].
In this study, we found that patients <50 years were more likely to have advanced stage EC. Several studies have shown high rates of stage III/IV disease in younger patients [Citation30–34]. Additionally, younger patients present more often with alarm symptoms, such as dysphagia (50–70) [Citation30–34]. In a recent analysis of Dutch primary care databases, it was concluded that a delayed diagnosis is most likely due to prolonged patient intervals (from first symptom to first presentation) [Citation35]. Lower cancer awareness and symptom recognition are thought to be responsible for prolonged patient intervals. Inefficiency in patient flows after presentation seems negligible in the Dutch setting as median lead times of primary to secondary care are relatively short [Citation35,Citation36]. Another hypothetical explanation for more advanced disease in young patients is a more aggressive tumor biology. Age-specific molecular mechanisms of carcinogenesis and tumor progression have not yet been established, but accelerated pathways of malignant progression of Barrett esophagus to EAC have been reported [Citation37].
Overall, all patients in the Netherlands benefited from important improvements in clinical staging, better patient selection, and centralisation of EC surgery [Citation5]. The nationwide initiation and implementation of the Dutch Upper GI Cancer Audit since 2011, which provides comparative and benchmarked information on surgical outcome, has further improved the quality of surgical care [Citation7]. Nonetheless, our long-term national data show an increasing survival difference between patients <50 years and ≥75 years with potentially curable or palliative disease. In 2018, the survival gap was almost 30% for potentially curable and 20% for palliative disease. Similar trends have been reported for colorectal and gastric cancer [Citation9,Citation38]. Differences in treatment choices between young and elderly patients may explain these survival gaps. Stage migration could have affected the survival outcomes per stage, but as we showed that relative survival also increased in all age categories unstratified for stage grouping, it is unlikely that stage migration was the main factor explaining the improved survival.
For early-stage EC, the five-year RSR of patients <50 years doubled over time and approached 100% in the period 2014–2018. The improved prognosis is mainly attributed to the successful introduction of endoscopic resection which has completely replaced surgery as an effective minimal-invasive curative modality with much lower morbidity [Citation39,Citation40].
The relative survival trend of potentially curable disease showed a steady increase in all age categories. Nonetheless, patients <50 years seem to have gained the largest survival benefit. The considerably higher rates of multimodality treatment use, including CROSS-based neoadjuvant chemoradiotherapy, in young patients may well explain the disproportionate survival gain between young and older patients.
In the palliative setting, survival improvement was also most prominent in patients <50 years. The proportion of palliative staged patients receiving any form of tumor-specific therapy increased over time in all age groups, i.e. up to 90% in patients <50 years in comparison to 50% in patients ≥75 years. This trend in young adults appears to be driven by the increased use of palliative treatment regimens that are primarily aimed at prolonging life expectancy. An increased use of external beam radiotherapy merely aimed at symptom relief and higher quality of life, and limited use of systemic therapy may explain the unchanged survival trend in older palliative patients.
This study is the first to present an overview of EC incidence rates in younger adults in Europe. Since the Netherlands Cancer Registry collects population-based data with almost 100% coverage of the cases, the registry allows for comprehensive and reliable estimations of epidemiological metrics such as incidence and survival. Limitations should also be acknowledged. Because information about cause of death was not available, net survival was estimated with relative survival analysis. Furthermore, it was not possible to adjust for factors known to impact choice of treatment and survival, such as performance status, comorbidities, or patient preferences. Lastly, no data were available on quality of life.
In conclusion, the incidence of EAC is increasing in adults <50 years. Particularly, in young females, EAC incidence is rising twice as fast as in males. Once diagnosed, young patients have more advanced disease stages but are also more likely to receive multimodality treatments with curative or life-prolonging intent compared to older patients. Patients <50 years experienced the most significant survival improvement over time, leading to substantial survival gaps between younger and older patients. Besides etiological studies on the modifying risk factors of EAC at young age, more studies are needed investigating the determinants of treatment decisions in different age groups. Furthermore, cancer prevention and awareness campaigns and intensified efforts to develop cost-effective and minimally invasive screening strategies for early detection of EC should also be prioritised in future studies.
Ethical approval
The study was approved by the scientific committees of the Dutch Upper GI Cancer Group and the Netherlands Cancer Registry. Patient consent was not applicable for this study.
Author contributions
PDS, RHAV, and AAK contributed to the conception and design of the study; AAK, NSB, and RHAV contributed to data acquisition, (statistical) analysis, interpretation and manuscript preparation. All authors contributed to drafting and revising the article.
| Abbreviations | ||
| CI | = | confidential interval |
| cN | = | clinical N stage |
| CRT | = | chemoradiotherapy |
| cT | = | clinical T stage |
| EAC | = | esophageal adenocarcinoma |
| EAPC | = | estimated annual percentage change |
| EC | = | esophageal cancer |
| ESCC | = | esophageal squamous cell carcinoma |
| IQR | = | interquartile range |
| pCR | = | pathological complete response |
| RSR | = | relative survival rate |
| SD | = | standard deviation |
| ESCC | = | esophageal squamous cell carcinoma |
Supplemental Material
Download MS Word (29.8 KB)Supplemental Material
Download PDF (1.2 MB)Disclosure statement
Rob HA Verhoeven has received research grants from Roche and Bristol-Myers Squibb. Hanneke van Laarhoven has served as a consultant for BMS, Celgene, Lilly, Nordic, Philips, and Servier and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, Roche, and Servier. Peter D. Siersema has received research grants from Boston Scientific, Pentax Medical, and The eNose company.
Data availability statement
The de-personalised data used in this study can be made available through application to the Netherlands Cancer Registry.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021; 71(3):209–249.
- Kamangar F, Nasrollahzadeh D, Safiri S, et al. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(6):582–597.
- Offman J, Pesola F, Sasieni P. Trends and projections in adenocarcinoma and squamous cell carcinoma of the oesophagus in England from 1971 to 2037. Br J Cancer. 2018; 118(10):1391–1398.
- de Vegt F, Gommers JJJ, Groenewoud H, et al. Trends and projections in the incidence of oesophageal cancer in The Netherlands: an age-period-cohort analysis from 1989 to 2041. Int J Cancer. 2022; 150(3):420–430.
- van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, et al. Long-term survival improvement in oesophageal cancer in The Netherlands. Eur J Cancer. 2018; 94:138–147.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366(22):2074–2084.
- Busweiler LA, Wijnhoven BP, van Berge Henegouwen MI, et al. Early outcomes from the Dutch upper gastrointestinal cancer audit. Br J Surg. 2016; 103(13):1855–1863.
- Wouters MW, Karim-Kos HE, Le Cessie S, et al. Centralization of esophageal cancer surgery: does it improve clinical outcome? Ann Surg Oncol. 2009; 16(7):1789–1798.
- Nelen SD, Verhoeven RHA, Lemmens V, et al. Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer. 2017; 20(6):919–928.
- Claassen YHM, Dikken JL, Hartgrink HH, et al. North European comparison of treatment strategy and survival in older patients with resectable gastric cancer: a EURECCA upper gastrointestinal group analysis. Eur J Surg Oncol. 2018; 44(12):1982–1989.
- Slagter AE, Tudela B, van Amelsfoort RM, et al. Older versus younger adults with gastric cancer receiving perioperative treatment: results from the CRITICS trial. Eur J Cancer. 2020; 130:146–154.
- Koeter M, van Putten M, Verhoeven RHA, et al. Definitive chemoradiation or surgery in elderly patients with potentially curable esophageal cancer in The Netherlands: a nationwide population-based study on patterns of care and survival. Acta Oncol. 2018; 57(9):1192–1200.
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017; 73:17048.
- Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185.
- Islami F, DeSantis CE, Jemal A. Incidence trends of esophageal and gastric cancer subtypes by race, ethnicity, and age in the United States, 1997–2014. Clin Gastroenterol Hepatol. 2019; 17(3):429–439.
- He H, Chen N, Hou Y, et al. Trends in the incidence and survival of patients with esophageal cancer: a SEER database analysis. Thorac Cancer. 2020; 11(5):1121–1128.
- Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 years. J Natl Cancer Inst. 2019; 111(12):1279–1297.
- Strauss A, Min EJ, Long Q, et al. Is the age of diagnosis of esophageal adenocarcinoma getting younger? Analysis at a tertiary care center. Dis Esophagus. 2020; 33(9): doz112.
- Guardino JM, Khandwala F, Lopez R, et al. Barrett's esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol. 2006; 101(10):2187–2193.
- Peters Y, Al-Kaabi A, Shaheen NJ, et al. Barrett oesophagus. Nat Rev Dis Primers. 2019; 5(1):35.
- Ezzati M, Henley SJ, Lopez AD, et al. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005; 116(6):963–971.
- Graham H. Smoking prevalence among women in the European Community 1950-1990. Soc Sci Med. 1996; 43(2):243–254.
- Jemal A, Miller KD, Ma J, et al. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med. 2018;378(21):1999–2009.
- Lewis DR, Check DP, Caporaso NE, et al. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883–2892.
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005; 242(4):566–573. discussion 573–575.
- Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide esophageal cancer collaboration: clinical staging data. Dis Esophagus. 2016; 29(7):707–714.
- Verbeek RE, Leenders M, ten Kate FJW, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population-Based cohort study. Am J Gastroenterol. 2014;109(8):1215–1222.
- Peters Y, Schrauwen RWM, Tan AC, et al. Detection of Barrett's oesophagus through exhaled breath using an electronic nose device. Gut. 2020;69(7):1169–1172.
- Ross-Innes CS, Debiram-Beecham I, O'Donovan M, on behalf of the BEST2 Study Group, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med. 2015;12(1):e1001780. Jan
- van Nistelrooij AM, van Steenbergen LN, Spaander MC, et al. Treatment and outcome of young patients with esophageal cancer in The Netherlands. J Surg Oncol. 2014; 109(6):561–566.
- Hamouda A, Forshaw M, Rohatgi A, et al. Presentation and survival of operable esophageal cancer in patients 55 years of age and below. World J Surg. 2010; 34(4):744–749.
- Portale G, Peters JH, Hsieh CC, et al. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg. 2004; 70(11):954–958.
- Hashemi N, Loren D, DiMarino AJ, et al. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci. 2009; 54(8):1708–1712.
- Boys JA, Oh DS, Lewis JS, et al. Esophageal adenocarcinoma in patients younger than 40 years: a two-decade experience at a public and private hospital. Am Surg. 2015; 81(10):974–978.
- van Erp NF, Helsper CW, Slottje P, et al. Time to diagnosis of symptomatic gastric and oesophageal cancer in The Netherlands: where is the room for improvement? United European Gastroenterol J. 2020; 8(5):607–620.
- Al-Kaabi A, Siersema PD. Reducing time to diagnosis in gastroesophageal cancer is key to further improve outcome. United Eur Gastroenterol J. 2020; 8(5):507–508.
- Contino G, Vaughan TL, Whiteman D, et al. The evolving genomic landscape of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2017; 153(3):657–673.e1.
- Brenner H, Bouvier AM, Foschi R, EUROCARE Working Group, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131(7):1649–1658.
- Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007; 65(1):3–10.
- Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc. 2014; 79(2):224–232.e1.