Abstract
Background
The base of tongue squamous cell carcinoma (BOTSCC) is mainly an HPV-related tumor. Radiotherapy (EBRT) ± concomitant chemotherapy (CT) is the backbone of the curatively intended treatment, with brachytherapy (BT) boost as an option. With four different treatment strategies in Sweden, a retrospective study based on the population-based Swedish Head and Neck Cancer Register (SweHNCR) was initiated.
Material and methods
Data on tumors, treatment and outcomes in patients with BOTSCC treated between 2008 and 2014 were validated through medical records and updated as needed. Data on p16 status were updated or completed with immunohistochemical analysis of archived tumor material. Tumors were reclassified according to the UICC 8th edition.
Results
Treatment was EBRT, EBRT + CT, EBRT + BT or EBRT + CT + BT in 151, 145, 82 and 167 patients respectively (n = 545). A p16 analysis was available in 414 cases; 338 were p16+ and 76 p16−. 5-year overall survival (OS) was 68% (95% CI: 64–72%), with76% and 37% for p16+ patients and p16− patients, respectively. An increase in OS was found with the addition of CT to EBRT for patients with p16+ tumors, stages II–III, but for patients with tumor stage I, p16+ (UICC 8) none of the treatment strategies was superior to EBRT alone.
Conclusion
In the present retrospective population-based study of BOTSCC brachytherapy was found to be of no beneficial value in curatively intended treatment. An increase in survival was found for EBRT + CT compared to EBRT alone in patients with advanced cases, stages II and III (UICC 8), but none of the regimes was significantly superior to EBRT as a single treatment modality for stage I (UICC 8), provided there was p16 positivity in the tumor. In the small group of patients with p16− tumors, a poorer prognosis was found, but the small sample size did not allow any comparisons between different treatment strategies.
Introduction
An increase in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) has been seen during the past decades, with a shift to patients of lower age and with less abuse of tobacco and alcohol than seen earlier in this subsite of HNSCC. This rise is attributed to patients with HPV (Human Papilloma Virus) positive tumors [Citation1]. Patients with HPV-positive tumors have been found to have better overall survival than patients with HPV-negative tumors, among which the majority of tobacco and alcohol users are found [Citation2].
The prognostic strength of the TNM staging classification in the UICC 7th edition (UICC 7) to discriminate for survival between patients with tumor stages I–IV was found to be poor, without any statistically significant difference in 5-year survival between stages I–III and stage IV [Citation3,Citation4]. The superior outcome for patients with p16 positive (p16+) tumors, the accepted surrogate marker for HPV positivity, compared to patients with p16 negative (p16−) tumors, warranted an adjustment of the TNM classification system in the UICC 8th edition (UICC 8), adding information on tumor biology with p16 status of the tumor [Citation5]. The consequence of using UICC 8 is a lower tumor stage for patients with p16+ tumors compared to patients with p16− tumors, albeit the same local tumor spread [Citation4,Citation6].
In Sweden, OPSCC is the second most common cancer among the head and neck carcinomas [Citation7]. The major subsite is the tonsillar region, followed by the base of tongue (BOTSCC) and, less often, other oropharyngeal subsites. There are arguments for single- or combination-modality therapies in the curatively intended treatment of BOTSCC, namely for radical radiotherapy (EBRT) with or without the addition of concomitant chemotherapy (CT) and with or without the addition of a brachytherapy boost (BT).
The benefit and incremental value of adding treatment modalities need to be examined, taking into account the risk of adding toxicity, both acute and late side-effects, as well as the utilization of healthcare resources.
Since 2008 all head and neck cancer cases in Sweden have been reported to the Swedish Head and Neck Cancer Register (SweHNCR), along with treatment data, treatment results, recurrences and survival [Citation7]. Concordance with the compulsory cancer reports to the National Cancer Register of the National Board of Health and Welfare is more than 99%.
Four different treatment strategies could be identified during the study period, with distinct geographical distributions associated with institutional traditions within the six Swedish healthcare regions, each with populations ranging from 0.9 to 2.4 million inhabitants, based on the Swedish population in 2020. Broadly described, treatments differed with respect to the addition of BT and/or CT. No prospective randomized trials have been published to elucidate solid treatment guidelines regarding the composition of treatment of patients with BOTSCC. It was considered important, therefore, to examine differences in treatment and to compare outcomes for patients with BOTSCC in an effort to layout common national guidelines.
In addition, there was a need to analyze outcome data according to the HPV status of the tumors, assessed with the surrogate marker p16.
Our data is reported according to the STROBE reporting guidelines for observational studies within the EQUATOR network (https://www.equator-network.org/reporting-guidelines/strobe)
Aim
The aim of this study was to analyze outcomes for patients with BOTSCC who have undergone curatively intended treatment, specifically with respect to treatment strategy and p16 status of the tumors.
Material and methods
Patients
All patients with previously untreated base of tongue squamous cell carcinomas from 2008 to 2014 (ICD-10: C0.1, C02.4) were identified through the SweHNCR (n = 589). Thirty-eight of these were treated with palliative intent. Only patients accepted for curatively intended treatment were included in this study leaving a total of 551 patients. However, in six patients full treatment was, for different reasons, not given and thus 545 patients were eligible for evaluation of outcome: 408 males, 137 females (). All patient records were reviewed and validated against data in the SweHNCR, with missing or incorrectly entered data completed and corrected as needed.
Table 1. Squamous cell carcinomas of the base of tongue (n = 545).
p16 analysis
Out of 545 tumors, p16 status was reported in 191cases. After inquiries were sent to the local pathology departments for analysis of the archived tumor specimens, an additional 223 tumors could be analyzed. The remaining 131 specimens had insufficient tumor material left, and their status was accounted for as ‘p16?’.
To assess the p16 status of tumors, immunohistochemistry was performed at the discretion of each department of pathology on paraffin-embedded, formalin-fixated tumor material using conventional IHC methods. Staining was regarded as positive (p16+) when >70% of the tumor cells were strongly positively stained for p16.
TNM
All cases were originally classified according to UICC 7 [Citation3] as part of the routine workup during the study period. Retrospectively a reclassification of the p16+ cases in concordance with UICC 8 was performed [Citation5]. The main outcome of this is a downstaging of N-status, as UICC 7 N1-2b turns into N1, UICC 7 N2c changes to N2, while UICC 7 N3 remains N3. For T the reclassification results in the merge of UICC T4a-b into T4. Following this, and the changes in UICC 8 of the distribution of T and N in p16+ oropharyngeal cancers, a downstaging in the clinical tumor stage is evident. In the few patients with p16− tumors a reclassification was not possible, as it is based upon the presence of extra-nodal invasion or not, a feature not possible to correctly assess retrospectively.
Treatment
Patients were treated according to existing local guidelines in the six healthcare regions with any one of four regimens: external radiotherapy only (EBRT) n = 151; EBRT + chemotherapy (concomitant chemotherapy with or without induction chemotherapy), (EBRT + CT) n = 145; EBRT + brachytherapy (EBRT + BT) n = 82; and EBRT + brachytherapy + CT (concomitant chemotherapy with or without induction chemotherapy) (EBRT + BT + CT) n = 167. Neck dissections performed within 6 months from the end of radiotherapy were regarded as a part of the primary curatively intended treatment. A total of 132 patients went through a modified radical neck dissection, mainly due to remaining palpable nodes after the primary treatment or to local treatment strategies (see next under Section ‘Brachytherapy’).
The treatment strategies in relation to stages according to UICC 7 and UICC 8 are shown in .
External beam radiotherapy
All radiotherapy was administered with Intensity Modulated Radiotherapy (IMRT) or Volumetric Arc Therapy (VMAT) The radiotherapy was mainly conventionally fractionated, with 2 Gy per fraction given once a day, 5 days a week. In those patients for which BT was not included, EBRT was given with a radical dose of approximately 68 Gy. In the majority of cases, namely 87%, the patients received between 66 and 70 Gy.
Brachytherapy
Brachytherapy was given with Pulsed Dose Rate (PDR) in three centers in three different healthcare regions. There were two discernable regimes for brachytherapy. The first regime used lower doses, between 8 and12 Gy, and conventionally fractionated external radiotherapy to a total dose of approximately 68 Gy. In the triplet combination, EBRT + BT + CT, the same brachytherapy treatment patterns were used.
The second brachytherapy regime used a higher brachytherapy dose of 35 Gy given in combination with accelerated fractionation of EBRT at 1.7 Gy twice daily to a total dose of 40.8 Gy. As a consequence of this low dose of EBRT, close to all node-positive patients (n = 57) underwent neck dissection (n = 51), resulting in a neck dissection rate of 89% for this group, which explains the total 62% neck dissection rate in the EBRT + BT group ().
Medical therapy
When anti-tumoral medical therapy was given, it was either with chemotherapy or with cetuximab. Chemotherapy was either given prior to EBRT ± BT as induction CT or concomitantly with EBRT, or both. Induction CT was given as a platin-based combination regime in 2–3 cycles prior to EBRT. Concomitant CT was delivered once weekly, either as a total cisplatin dose of 50 mg or 40 mg/m2 with a maximum dose of 70 mg. When cetuximab replaced cisplatin, a loading dose of 400 mg/m2 was given, followed by 250 mg/m2 weekly.
Of the 312 patients who received chemotherapy, 55 patients received CT as induction therapy, 176 as concomitant CT + EBRT, and 65 patients both induction CT and concomitant CT + EBRT. Of these 65 patients, 55 received EBRT + BT + CT and the remaining 10 patients EBRT + CT. In 16 patients the timing of the given CT treatment was not stated (induction or concomitant).
In a total of 86 patients, 33 in the EBRT + CT treatment group and 53 patients in the EBRT + BT + CT treatment group, cetuximab replaced conventional chemotherapy.
Statistical methods
Analysis of overall survival was performed using the Kaplan-Meier method and differences in survival rates with the log-rank test. Time was calculated from the date of diagnosis to date of death or end of follow-up (1 June 2019). Differences in distribution between groups were tested using Fisher’s exact test. Uni- and multivariable Cox regression analyses for 5-year overall survival were used to analyze the association between different variables and their possible impact on OS.
Multiple imputations were used in the Cox regression analyses of difference in overall survival between patients treated with and without chemotherapy adjusting for age, WHO performance status classification, and smoking habits. A p-value <0.05 was considered statistically significant. All statistical analyses were carried out with Stata/IC 16.1 for Mac (StataCorp. 2020. Stata: Release 16. Statistical Software. College Station, TX: StataCorp LLC).
Results
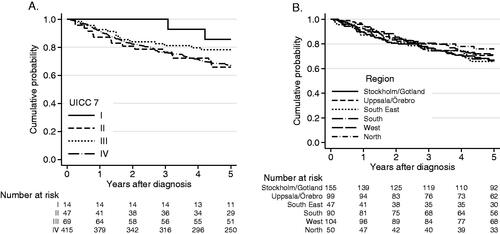
In the total cohort of 545 patients, the 5-year OS was 68% (95% CI: 64–72%), with 86%, 66%, 76% and 67% for BOTSCC stages I–IV, respectively (stage IV corresponds to stages IVA and B, UICC 7) (). Disease-free survival (DFS) at 5-years was 67%, with missing data in 17 patients.
Figure 1. (A) Overall survival in all patients with BOTSCC (n = 545) treated with a curative intent 2008–2014, in relation to tumor stage (UICC 7). Log-rank test p = 0.17. OS 5 years: Stage I: 86%, II: 66%, III: 78%, IV: 67%. (B) Overall survival (OS) for all patients with BOTSCC (n = 545) depending on residency, in the different health care regions. OS 5 years: Stockholm, South, South East: 66%, U/Ö, West: 70%, North: 75%. Log-rank test, p = 0.80.
In 414 tumors p16 status was available, resulting in 338 patients (82%) with p16+ tumors and 76 patients (18%) with p16− tumors. The fraction of p16+ tumors is considerably higher than reported for oropharyngeal SCC from, for example, the Netherlands [Citation8] and is more in line with figures reported from the United States and Canada [Citation9,Citation10].
The four different treatment approaches from all six treating centers showed equally good results, with a 5-year OS of 66% to75% (). The results appearing in are similar, showing OS results for stages I–IV (UICC 7) of 64–75% depending on treatment regimens, with no statistically significant differences depending on the regimens seen in a Cox regression analysis (). Accordingly, the DFS did not differ between the groups, with a 5 years DFS of 70%, 74%, 75% and 74% for patients treated with EBRT, EBRT + BT, EBRT + CT and EBRT + BT + CT, respectively (data not shown), with missing data on locoregional control (LRC) in 5 patients.
Figure 2. (A) Overall survival according to treatment strategy, for patients with tumor stages I–IV (UICC 7, n = 545). OS 5 years: EBRT: 64%, EBRT + BT: 71%, EBRT + CT: 65%, EBRT + BT + CT: 75%. (B) Overall survival and p16 analysis, p16? = not available for immunohistochemistry. Log-rank test, p < 0.001. OS 5 years: p16+: 77%, p16−: 38%, p16?: 66%.
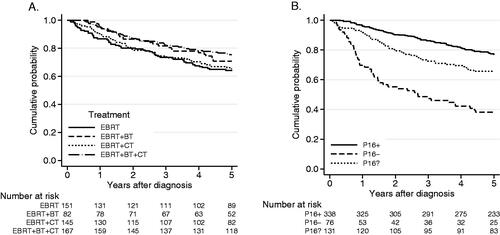
Table 2. Uni- and multivariable Cox regression.
As expected, patients with p16+ tumors fared better than those with p16− tumors, with 5-year survival figures of 77% and 38% respectively. In patients with p16? tumors, a 5-year OS of 66% (95% CI: 57–73%) was seen, a result comparable with survival figures for the whole study group. Representation of p16+ and p16− cases in the p16? group can therefore be assumed to be comparable to the whole study group (). For patients with p16+ tumors 5-year DFS was 73%, albeit missing data on LRC in 5 patients, compared to the 5-year DFS of 67% in the whole study group.
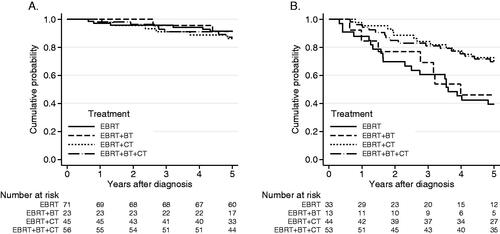
When analyzing OS for patients with stage I (UICC 8) p16+ tumors (n = 195), no gain in survival was seen with EBRT + CT or EBRT + BT + CT compared to EBRT alone (). For patients with p16+ tumors, stages II–III (UICC 8) (n = 143), the addition of CT to EBRT ± BT showed a gain in survival, with a 5-year OS of 70% compared to 43% without CT (). This result was supported by a Cox regression analysis, with a hazard ratio (HR) in univariate and multivariate analyses of 0.39 (95% CI: 0.23–0.66, p < 0.001) and 0.43 (95% CI: 0.28–0.85, p = 0.010) respectively, adjusted for age and smoking in the multivariate analysis. Younger patients and smokers were more likely to receive chemotherapy than older patients and nonsmokers. WHO performance grade was not a confounding factor.
Figure 3. (A) Overall survival for patients with p16+, stage l (UICC 8), according to treatment-strategy (n = 195). Log-rank test, p = 0.81. OS 5 years: EBRT: 92%, EBRT + BT: 91%, EBRT + CT: 86%, EBRT + BT + CT: 87%. (B) Overall survival for patients with p16+ BOTSCC, stages II–III, (UICC 8, n = 143) according to treatment-strategy. Log-rank test, p = 0.004. OS 5 years: EBRT: 39%, EBRT + BT: 46%, EBRT + CT: 70%, EBRT + BT + CT: 69%.
For patients with p16− tumors (n = 76), regardless of stage, a poor prognosis was found, but the small sample size does not allow any definitive comparisons between different treatment strategies.
Discussion
This retrospective study of BOTSCC diagnosed in Sweden between 2008 and 2014 revealed similar survival rates in the six healthcare regions in Sweden. This result was seen despite pronounced differences in treatments, mainly regarding the addition of chemotherapy and brachytherapy to external beam radiotherapy. The excellent conformity between the national cancer register and the register for head and neck allows robust conclusions to be drawn and enables uniform national treatment guidelines to be agreed upon.
With regard to p16 status, a subsequent reclassification from UICC 7 to UICC 8 revealed differences in outcome depending on tumor stage and treatment strategy. A benefit was noted in survival for patients with stages II–III (UICC 8), p16+ tumors, who received concomitant chemoradiotherapy compared to radiotherapy alone. The addition of brachytherapy did not convey any gain in survival. In 24% of the patients, p16 could not be analyzed. However, there is no reason to assume any accidental selection bias.
The gold standard for determining the presence of high-risk strands of human papillomavirus in OPSCC is the detection of viral mRNA, with PCR-based detection of viral DNA secondary [Citation11]. Since mRNA analysis requires fresh tissue while PCR-based detection of HPV DNA can be performed on formalin-fixed paraffin-embedded samples, the latter technique is usually used. Data from SweHNCR (unpublished data) with 264 paired comparisons showed very good concordance between PCR-based detection of viral HPV and the p16 assay, with a sensitivity of 98% and a specificity of 89%. This result contrasts with findings of low concordance between p16 and HPV DNA, with a sensitivity of 49% in non-tonsillar, non-base of tongue OPSCC [Citation12]. Thus, it must be kept in mind that findings from studies of BOTSCC cannot be applied to all OPSCC.
The strength of this study is that it is based on a population-based cancer register with a coverage of nearly 100%. Consecutive cases treated with curative intent during the defined time period were analyzed. Moreover, all data from the register were subsequently validated from medical records. Given the validated treatment and outcome data, the results will affect treatment guidelines nationally for HNSCC.
The retrospective nature of this study gives rise to limitations. A selection bias favoring chemotherapy for younger patients was seen. For patients with p16− tumors, the number of patients was not large enough to analyze treatment outcomes for different treatments.
Radiotherapy doses were validated, but with medical treatment, there is uncertainty regarding the number of weekly chemotherapy cycles. Treatment side effects could not be analyzed, neither acute nor late. Quality of life following these demanding treatments could not be reported. No health care economic analysis was carried out, but differences in treatment costs and morbidity are to some extent obvious depending on the inclusion or not of CT and/or BT. It is clear that treatment costs are decreased and quality of life increased in the group of patients with p16+ BOTSCC stage I when chemotherapy and brachytherapy can be omitted.
A prospective randomized controlled trial (RCT) is clearly superior to an observational study but real-world observational studies with validated national data have both legitimacy and strengths, as is discussed by Benson & Hartz and by Bergqvist et al. [Citation13,Citation14].
Brachytherapy as a part of a combined treatment approach did not convey any benefit compared to strategies without brachytherapy. Recently Silva et al. presented a retrospective database study of 28,317 patients with BOTSCC treated with radiotherapy [Citation15]. The vast majority of the patients (27,954) received ERBT alone, 154 received BT alone, and 209 patients were treated with a combination of ERBT and BT. Treatment with EBRT ± concomitant CT ± BT compared to BT alone was considered with respect to 3-year OS. A superior outcome was found for the group of patients treated with EBRT + BT, with a 3-year survival of 77.1% compared to 69.6% for patients treated with EBRT and 63.7% with BT alone. Firm conclusions are hard to state as the groups were heavily unbalanced, with only 209 in the EBRT + BT group and 154 in the BT alone group. Furthermore, in the majority of patients, the HPV-status of the tumor was unknown. BT could, however, be a tool in selected situations, such as for recurrences [Citation16].
Concomitant chemoradiotherapy, EBRT + CT, has been the internationally recommended treatment for many years, for locoregionally advanced cases [Citation17]. In concordance, our study shows that concomitant chemoradiotherapy is of benefit for survival in patients with p16+ tumors and locally advanced disease corresponding to stages II–III (UICC 8), that is, with either a very large primary tumor or advanced regional lymph node invasion.
With the increase in OPSCC in younger patients with a strikingly better treatment outcome in HPV-associated tumors, frequent proposals have been made to de-escalate the treatment of patients with p16+ tumors. In two reviews [Citation18,Citation19] the topic of de-escalated treatment in HPV-associated OPSCC was discussed. Studies replacing concomitant cisplatin with cetuximab have failed both in respect to survival and toxicity, but administration of cisplatin weekly instead of high-dose cisplatin every third week has shown encouraging results [Citation20].
At the same time, patients with advanced disease, T4 or N3, and patients with low neck disease seem to be at risk for late recurrences and distant disease and might benefit from intensified treatments. The NRG-HN002 randomized Phase II study comparing EBRT 60 Gy ± CT in p16+ OPSCC, stages III–IV (UICC 7), with EBRT alone, found with the same 2-year OS of 97% but with poorer 2-year progression-free survival in the EBRT-only arm [Citation21]. Yoshida et al. found in a registered study using the United States National Cancer Database a small but statistically significant increase in survival for patients with stage I (UICC 8) HPV-associated disease treated with EBRT + concomitant chemotherapy compared to EBRT alone, but only for patients with the node-positive disease [Citation22]. This is in concordance with our findings of excellent treatment outcomes with EBRT alone in p16+ BOTSCC, also for node-positive patients, for stage I.
In patients with p16− tumors exhibiting very poor survival, there is a demand to improve outcomes. The demographic differences that have been noted, with more smokers, poorer WHO performance status, and higher ages among patients with p16− tumors compared to the p16+ counterparts can partly explain poorer outcomes, but a decreased sensitivity to radiotherapy has also been reported [Citation23].
In the light of the vast literature dealing with disparities in clinical findings and outcomes between HPV-associated versus not HPV-associated OPSCC, a challenge currently exists to define treatment guidelines appropriate for different subgroups. In a chart review by Jaber et al., the incidence and pattern of dissemination varied depending on p16 status, with distant metastases more frequent and more widely spread in patients with p16+ tumors compared to their p16− counterparts, suggesting hematologic spread [Citation24]. This finding challenges the ideas of de-escalating treatment in patients with p16+ tumors and warrants further studies to identify subgroups of patients for which systemic treatment could convey survival benefits.
Conclusion
In the present retrospective population-based study of BOTSCC, brachytherapy was found to be of no beneficial value in curatively intended treatment. An increase in survival was found for EBRT + CT compared to EBRT alone in patients with advanced cases, stages II and III (UICC 8), while none of the regimes was significantly superior to EBRT as a single treatment modality for stage I (UICC 8), provided p16 positivity in the tumors. In the small group of patients with p16− tumors a poorer prognosis was found, but the small sample size did not allow for any comparisons between different treatment strategies.
Ethical approval statement
The present work was ethically approved by Gothenburg 299-14 and T230-17.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are obtained from the national quality register for head and neck cancer, SweHNCR, for the study after ethical approval and approval from the registration authority.
Additional information
Funding
References
- Attner P, Du J, Nasman A, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126(12):2879–2884.
- Attner P, Du J, Nasman A, et al. Human papillomavirus and survival in patients with base of tongue cancer. Int J Cancer. 2011;128(12):2892–2897.
- Sobin LH, Gospodarowicz MK, Wittekind C, et al. TNM classification of malignant tumours. 7th ed. Chichester (UK); West Sussex (UK); Hoboken (NJ): Wiley-Blackwell; 2010.
- van Gysen K, Stevens M, Guo L, et al. Validation of the 8(th) edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo-radiotherapy. BMC Cancer. 2019;19(1):674.
- Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Chichester (UK); West Sussex (UK); Hoboken (NJ): John Wiley & Sons, Inc.; 2017.
- Wurdemann N, Wagner S, Sharma SJ, et al. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017;7:129.
- SweHNCR. The Swedish Head and Neck Cancer Register. Gothenburg (Sweden): Regional Cancer Centre West; 2020. https://www.cancercentrum.se/samverkan/cancerdiagnoser/huvud-och-hals/kvalitetsregister.
- Nauta IH, Rietbergen MM, van Bokhoven A, et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in The Netherlands and the importance of additional HPV DNA testing. Ann Oncol. 2018;29(5):1273–1279.
- Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9):1968–1975.
- O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the international collaboration on oropharyngeal cancer network for staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440–451.
- Bussu F, Ragin C, Boscolo-Rizzo P, et al. HPV as a marker for molecular characterization in head and neck oncology: looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck. 2019;41(4):1104–1111.
- Hammarstedt L, Holzhauser S, Zupancic M, et al. The value of p16 and HPV DNA in non-tonsillar, non-base of tongue oropharyngeal cancer. Acta Otolaryngol. 2021;141(1):89–94.
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886.
- Bergqvist D, Bjorck M, Sawe J, et al. Randomized trials or population-based registries. Eur J Vasc Endovasc Surg. 2007;34(3):253–256.
- Silva SR, Martin B, Choi M, et al. National cancer database analysis of the effect of brachytherapy on overall survival in patients with base of tongue cancer. Head Neck. 2019;41(5):1184–1192.
- Bussu F, Tagliaferri L, Mattiucci G, et al. HDR interventional radiotherapy (brachytherapy) in the treatment of primary and recurrent head and neck malignancies. Head Neck. 2019;41(6):1667–1675.
- Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33–40.
- Patel RR, Ludmir EB, Augustyn A, et al. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: a systematic review of prospective trials. Oral Oncol. 2020;103:104608.
- Price KAR, Nichols AC, Shen CJ, et al. Novel strategies to effectively de-escalate curative-intent therapy for patients with HPV-associated oropharyngeal cancer: current and future directions. Am Soc Clin Oncol Educ Book. 2020;40:1–13.
- Gebre-Medhin M, Brun E, Engstrom P, et al. ARTSCAN III: a randomized phase III study comparing chemoradiotherapy with cisplatin versus cetuximab in patients with locoregionally advanced head and neck squamous cell cancer. J Clin Oncol. 2021;39(1):38–47.
- Yom SS, Torres-Saavedra P, Caudell JJ, et al. NRG-HN002: a randomized phase II trial for patients with p16-positive, non-smoking-associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol. 2019;105(3):684–685.
- Yoshida EJ, Luu M, Mallen-St Clair J, et al. Stage I HPV-positive oropharyngeal cancer: should all patients receive similar treatments? Cancer. 2020;126(1):58–66.
- Ozcan-Wahlbrink M, Schifflers C, Riemer AB. Enhanced radiation sensitivity of human papillomavirus-driven head and neck cancer. Front Immunol. 2019;10(2831):2831.
- Jaber JJ, Murrill L, Clark JI, et al. Robust differences in p16-dependent oropharyngeal squamous cell carcinoma distant metastasis: implications for targeted therapy. Otolaryngol Head Neck Surg. 2015;153(2):209–217.
