Background
The Dutch Stop & Go study (BOOG 2010-02) compared an interrupted chemotherapy schedule with pre-defined breaks in treatment to a standard continuous schedule for patients with advanced HER2-negative breast cancer [Citation1]. The Stop&Go study assumed a noninferiority margin of 1.34 for the upper limit of the 95% confidence interval (CI) for the hazard ratio (HR) of the primary outcome first-line progression-free survival (PFS). Such non-inferiority could not be confirmed, with a median first-line PFS of 7.4 months for intermittent, compared with 9.7 months (HR 1.17; 95%CI 0.88–1.57) for continuous scheduling [Citation1]. Additionally, secondary analysis of the overall survival (OS) showed a benefit in favor of continuous scheduling (medians 17.1 vs. 20.9 months, HR 1.37; 95%CI 1.03–1.84) [Citation2]. Furthermore, no clear difference in QoL was found [Citation3]. Although we could not show that intermittent treatment was noninferior to continuous treatment in terms of (progression-free) survival outcomes, it could still be that intermittent treatment has a favorable societal impact, if the relative small reduction of the effectiveness comes with a considerable saving of costs. A Dutch study found an increase in total healthcare costs for breast cancer from €199 million in 2003 to €692 million in 2011 [Citation4]. These costs are highest for the more advanced stages of breast cancer, [Citation5–7] in the final year of life, [Citation8–12] and after progression of disease, [Citation7,Citation10,Citation11] underlining the importance of (cost)effective treatment management of advanced breast cancer (ABC). In order to explore this line of reasoning further, we examined the cost-effectiveness of continuous versus intermittent treatment for patients with locally advanced incurable or metastatic HER2-negative breast cancer who had not received chemotherapy for advanced disease.
Methods
This cost-effectiveness study examines whether the additional costs of continuous treatment (standard of care) are worth the additional health benefits compared to intermittent treatment, based on data collected in the Stop&Go study.
Patient selection from the Stop&Go study
Data regarding OS, QoL, and resource utilization were collected in the phase III Stop&Go study [Citation1]. The Stop&Go study included patients (N = 420) with locally advanced incurable or metastatic HER2-negative breast cancer who had not received chemotherapy for advanced disease. Participants were randomized to intermittent chemotherapy (two times four cycles; second set of four cycles of the same regime in case of progression after at least 3 months) or continuous chemotherapy (one set of eight cycles), both in first- and second-line treatment. First-line chemotherapy comprised paclitaxel, which was combined with bevacizumab that was continued as maintenance treatment, also during the chemotherapy break in the intermittent group. Second-line treatment comprised either capecitabine or non-pegylated liposomal doxorubicin. Patients who fulfilled at least one QoL measurement were selected for the present economic analysis (N = 402, 96% of total).
Cost collection
The economic assessment was based on study-related, direct medical costs made during study-treatment. We collected medical study costs over a maximum period of 24 months after randomization as we presumed on forehand that most patients would have completed study treatment within this timeframe; that is would have stopped with either the original continuous or intermittent therapy scheme, and either started follow-up treatments or did not receive further anti-tumor treatments. Costs generated after end of study-treatment and indirect costs were not included. A total of 12 patients in the continuous and 14 patients in the intermittent treatment group generated study-related costs beyond 24 months (total 6.5% of study population), which were not included in these analyses. Details on data collection and prizing can be found in the Online Supplement.
Statistical analyses
Mean life years per treatment arm were estimated by calculating the total number of Life Years (LY) between randomization and death or 24 months after randomization, whichever came first. The difference between the groups represented the LY gained.
Quality-Adjusted Life-Years (QALYs) were calculated as a measure of overall health benefit from the available QoL data as measured by the 36-Item RAND Health Survey. The RAND-36 was administered by post, at baseline and at regular intervals of 3 months [Citation3]. Time horizon was 24 months, calculated beyond date of randomization. To estimate QALYs, we applied the syntax provided by Brazier (personal communication and Brazier et al. [Citation13]) to convert QoL data as measured by the RAND-36 questionnaires to so called ‘utility scores’, rated on a scale where 0 reflects a state equal to dead and 1 to full health. For this procedure 11 of the 36 questionnaire items were used [Citation13]. Further information on statistical methods for calculation of QALY’s can be found in the Online Supplement.
Differences in several costs (medication, hospital visits, disease assessments, concurrent non-study anti-tumor treatment, and total costs) were analyzed with Mann–Whitney U-tests. Cost-effectiveness was quantified using Incremental Cost-Effectiveness Ratios (ICERs) in €per QALY and €per LY gained [Citation14,Citation15]. ICERs were calculated as the quotient of the difference in costs and QALYs or LYs gained. Calculated QALYs, LYs and costs were bootstrapped using sampling with replacement, using 10,000 samples. The outcomes were graphically depicted in a scatterplot to visualize the uncertainty of the estimates regarding the incremental costs and incremental benefits. In this graph, the willingness-to-pay threshold was also included. This refers to the amount a society is willing to pay to obtain 1 QALY (i.e., 1 additional year in full health) [Citation16]. The Dutch willingness-to-pay threshold for severe diseases is established at €80,000/QALY [Citation17]. Next, the probability that continuous therapy is cost-effective compared to intermittent therapy was graphically represented using cost-effectiveness acceptability curves (CEAC) [Citation18].
Statistical analyses were performed with SPSS software (version 23; IBM Corp, Armonk, NY, USA). P-values lower than 0.05 were considered significant. R (version 4.0.1) was used for bootstrapping.
Sensitivity analyses
As previous studies found no OS benefits of adding bevacizumab to paclitaxel chemotherapy, [Citation19,Citation20] current international guidelines for treatment of ABC recommend to consider bevacizumab in combination with chemotherapy only in selected cases [Citation21]. To explore uncertainty of our data, we post hoc subjected use of bevacizumab to sensitivity analyses. We estimated costs and ICERs in case bevacizumab would not have been used in the Stop&Go study, assuming similar outcomes.
Results
A total of 402 patients were found eligible for the current analyses (i.e., fulfilled at least one QoL measurement). A total of 2201 utility scores were calculated out of these questionnaires, with 68 missing utility scores (>2 out of the required 11 items missing).
Clinical outcomes
Baseline characteristics of the 402 eligible patients were well-balanced between randomized groups (Online Supplement Table 1).
Table 1. Costs per patient during Stop&Go study-treatment per treatment arm (maximum up to 24 months after randomization), indexed to 2019 cost prices.
Cost-effectiveness analysis
The estimated mean QALYs were 0.912 (SD ±0.44) and 0.891 (SD ±0.47) for continuous and intermittent treatment, respectively. Mean utility (estimated through dividing QALY by LYs) for QoL was 0.603 for the continuous and 0.614 for the intermittent group.
Average costs per patient, accumulated during the 24 months study period, were €65,740 for the continuous and €61,290 for the intermittent treatment group, leading to incremental costs of €4450 for the continuous treatment. Cost drivers (>€5000 per patient) were in descending order: treatment with bevacizumab, planned hospital visits for administration of study-treatment, treatment with non-pegylated liposomal doxorubicin, hospitalizations (overnight stays), and radiotherapy (). Consistently, the bootstrapped results indicated that continuous treatment would be more effective (survival and QALY gains of 0.061 and 0.021, respectively) and more costly (€4.454 additional costs) than intermittent treatment. This resulted in ICERs of €72,614 and €210,140 per LY and per QALY gained respectively () for continuous versus intermittent therapy.
Table 2. Cost-effectiveness results (based on bootstrap).
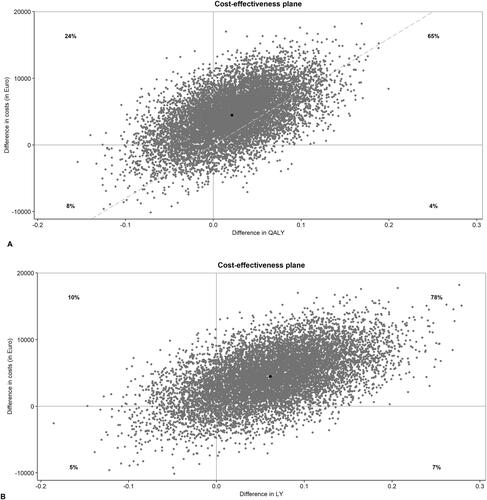
represents the individual point estimates of the ICERs of all 10,000 bootstrapped samples in an incremental cost-effectiveness plane. Most dots (65%) of the QALY-plane are plotted in the upper right quadrant, indicating that continuous treatment generates more QALYs but is also more costly than intermittent therapy. Likewise, within the LY-plane the majority (78%) of the dots are in the upper right quadrant, where continuous treatment generates more LYs but is more costly than intermittent therapy.
Figure 1. Cost-effectiveness planes of individual point estimates of the ICERs for QALY (A) and LY (B) gained of all 10,000 bootstrapped samples. The incremental costs and incremental benefits are demonstrated for continuous over intermittent treatment. The Y-axis expresses costs, where positive values indicate incremental costs for continuous therapy and negative values indicated incremental costs for intermittent therapy. On the X-axis QALYs (fig A) and LYs (fig B) are expressed, with positive values indicating benefits in these outcomes for continuous treatment and negative values indicating benefits for intermittent treatment respectively.
ICERs: Incremental cost-effectiveness ratios; QALY: Quality-Adjusted Life-Year; LYs: life year.
The probability that continuous treatment is cost-effective over intermittent therapy at the national willingness-to-pay level of €80,000/QALY is 21.8%. Results are displayed in an cost-effectiveness acceptability curve (CEAC) in Online Figure 1.
Sensitivity analysis
Without the costs for bevacizumab while assuming comparable outcomes, total costs would be €17,140 for continuous and €14,239 for intermittent treatment, leading to incremental costs of €2901 and ICERs of €47,557/LY and €138,143/QALY gained respectively ().
Discussion
The present cost-effectiveness analyses of data from the Stop&Go study within the first 24 months indicates small survival as well as QALY gains with continuous chemotherapy scheduling of eight consecutive cycles compared to an interrupted schedule of two times four cycles in first- and second line treatment in patients with advanced HER2-negative breast cancer. However, these small benefits of continuous therapy do not seem to offset the additional costs: with ICERs of €72,614/LY gained and €210,140/QALY gained, the continuous chemotherapy strategy cannot be considered cost-effective compared with intermittent therapy, considering the Dutch national willingness-to-pay threshold of €80,000/QALY.
Previous studies [Citation22], including earlier publications of the Stop & Go study [Citation1,Citation2], concluded to continue chemotherapy as long as possible up to 8 cycles. Within the Stop&Go study, median OS for all 420 randomized patients was respectively 20.9 versus 17.1 months for continuous versus intermittent treatment [Citation2]. According to the Dutch national committee for assessment of oncological agents, this benefit in OS of more than 12 weeks is considered effective [Citation23]. Additionally, several studies showed that QoL outcomes were not harmed by longer durations of (consecutive) chemotherapy treatment [Citation3,Citation24], Here, we report small gains in QALY’s and LY gained with continuous treatment within a time horizon of 24 months that did not outweigh the small mean additional costs of €4450. It should be noted that earlier reports from the Stop&Go trial presented analyses based on data beyond 24 months [Citation1–3]. As we did not have sufficient quality costs data beyond 24 months, and because the interpretation becomes more and more difficult due to different follow up treatments, we truncated the analysis at 24 months. If the studied time horizon would be extended, the survival gains of continuous treatment would potentially increase, increasing the estimated QALYs and likely improving its cost-effectiveness. Furthermore, the fact that differences in QALY’s and LY gained found here were small, indicates that based on previously published (clinical) outcomes the continuous chemotherapy strategy should be preferred.
The major cost-driver in our analysis was the use of bevacizumab () while in current clinical practice bevacizumab is only recommended by international guidelines to be considered in combination with chemotherapy in selected cases [Citation21]. Additionally, the use of bevacizumab in combination with first-line chemotherapy was already reduced after the FDA provoked their approval in 2011 [Citation25] and will probably further decline due to the recent introduction of new treatment options for ABC. We performed post hoc sensitivity analyses leaving out the costs of bevacizumab treatment. These analyses generated ICERs of €47,557/LY and €138,143/QALY for continuous over intermittent treatment, a ratio closer to the national willingness-to-pay threshold of €80,000/QALY. Noteworthy, bevacizumab will be out of patent in the near future which may thus impact our ICER results [Citation26].
Despite large discrepancies in methods used, other studies in ABC patients receiving comparable therapy report similar health-state utility scores. Specifically, a Dutch cost-effectiveness study on the use of bevacizumab in HER2-metastatic breast cancer, partly using the same treatment agents and within the same country as our study, reported cross-sectional EQ-5D health-state utilities in the real-world population of 0.66 and 0.55 for the progression-free and progressive disease health states respectively [Citation27]. Dedes et al. used utility scores of 0.61 for stable disease and 0.26 for progressive disease in their Markov model-based cohort simulation for ABC patients treated with bevacizumab and paclitaxel [Citation28].
Conclusion
Our results suggest that in advanced HER2-negative breast cancer patients, continuous chemotherapy in first- and second-line, cannot be considered cost-effective compared to intermittent chemotherapy. However, results were largely influenced by the costs of bevacizumab when taking the sensitivity results into account. Therefore, we recommend to guide chemotherapy duration primarily on clinical effectiveness and quality of life rather than on cost aspects.
Supplemental Material
Download MS Word (94.1 KB)Acknowledgements
We thank all patients, physicians (research)nurses and local data managers who participated in the Stop&Go trial; the members of the steering committee, the data safety monitoring committee, the central data management from the Comprehensive Cancer Centre the Netherlands, especially Steffen de Groot; and the Dutch Breast Cancer Research Group. We thank the teams from F. Hoffmann-La Roche Ltd and TEVA Nederland B.V. for their support.
Disclosure statement
FE has received honoraria from Roche and Novartis and has a consulting/advisory role for these companies. EvL has received research funding for her institution from Roche and TEVA. VTH has received honoraria and/or travel, accommodations and/or expenses from Pfizer, E. Lilly, Novartis and Roche, has a consulting or advisory role for Pfizer, E. Lilly, Novartis and Roche, has received research funding for her institution from Roche, Eisai, Pfizer, E. Lilly, Novartis, and AstraZeneca. MB has received Travel, accommodations and/or expenses from Roche, Novartis and Pfizer. All remaining authors have declared no conflicts of interest.
Data availability statement
Additional data on the trial protocol can be found at the EU Clinical Trials Register, using number 2010-021519-18 (https://www.clinicaltrialsregister.eu/ctr-search/search?query=2010-021519-18). The datasets generated and analyzed during the current study are not publicly available due to confidential cost-prices. Data are however are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Claessens AKM, Bos M, Lopez-Yurda M, et al. Intermittent versus continuous first-line treatment for HER2-negative metastatic breast cancer: the stop & go study of the Dutch breast cancer research group (BOOG). Breast Cancer Res Treat. 2018;172(2):413–423.
- Claessens AKM, Erdkamp FLG, Lopez-Yurda M, Dutch Breast Cancer Research Group (BOOG), et al. Secondary analyses of the randomized phase III stop&go study: efficacy of second-line intermittent versus continuous chemotherapy in HER2-negative advanced breast cancer. Acta Oncol. 2020;59(6):713–722.
- Claessens AKM, Timman R, Busschbach JJ, et al. The influence on quality of life of intermittent scheduling in first- and second-line chemotherapy of patients with HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2020;179(3):677–685.
- Vondeling GT, Menezes GL, Dvortsin EP, et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18(1):262.
- Broekx S, Den Hond E, Torfs R, et al. The costs of breast cancer prior to and following diagnosis. Eur J Health Econ. 2011;12(4):311–317.
- Campbell JD, Ramsey SD. The costs of treating breast cancer in the US: a synthesis of published evidence. Pharmacoeconomics. 2009;27(3):199–209.
- Lamerato L, Havstad S, Gandhi S, et al. Economic burden associated with breast cancer recurrence: findings from a retrospective analysis of health system data. Cancer. 2006;106(9):1875–1882.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117–128.
- Haas LC, Fernandes RA, Bines J, et al. Hormonal receptor positive, HER2 negative metastatic breast cancer (MBC HR + HER2-): pre and Post-Progression costs under the public health care system (SUS) and societal perspectives in Brazil. Value in Health. 2013;16(7):A404.
- Allen JM. Economic/societal burden of metastatic breast cancer: a US perspective. Am J Manag Care. 2010;16(9):697–704.
- Frederix GW, Severens JL, Hovels AM, et al. Time-dependent resource use and costs associated with different states of disease in patients diagnosed with HER-2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;139(2):489–495.
- Rao S, Kubisiak J, Gilden D. Cost of illness associated with metastatic breast cancer. Breast Cancer Res Treat. 2004;83(1):25–32.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economical evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Blank PR, Dedes KJ, Szucs TD. Cost effectiveness of cytotoxic and targeted therapy for metastatic breast cancer: a critical and systematic review. Pharmacoeconomics. 2010;28(8):629–647.
- Barshes NR, Chambers JD, Cantor SB, et al. A primer on cost-effectiveness analyses for vascular surgeons. J Vasc Surg. 2012;55(6):1794–1800.
- Zwaap J, Knies S, van der Meiden C, et al. Guideline for economic evaluations in healthcare. In: Institute NHC, editor. 2015. p. 1–40.
- Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415.
- Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247.
- Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260.
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657.
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol. 2011;29(16):2144–2149.
- PASKWIL-criteria 2016: palliatieve behandeling [Internet]. Nationale vereneging voor medische oncologie (NVMO). 2016. Available from: https://www.nvmo.org/over-de-adviezen/.
- Claessens AKM, Ibragimova KIE, Geurts SME, et al. The role of chemotherapy in treatment of advanced breast cancer: an overview for clinical practice. Crit Rev Oncol Hematol. 2020; 153(153):102988.
- van Kampen RJW, Lobbezoo DJA, de Boer M, et al. A real-world study on implementation of bevacizumab in a cohort of HER2-negative metastatic breast cancer patients: a study of the southeast Netherlands breast cancer consortium. Cancer Treat Res Commun. 2017; 13:3–8.
- ZorgInstituutNederland(DutchHealthcareAuthority). Bevacizumab 2019 [updated 2019 Jun 12; cited 2021 Oct 5]; Version 1:[Description of agent and biosimilars, expected approval]. Available from: https://www.horizonscangeneesmiddelen.nl/geneesmiddelen/bevacizumab-oncologie-en-hematologie-oncologie-overig%5B2%5D/versie1.
- van Kampen RJW, Ramaekers BLT, Lobbezoo DJA, et al. Real-world and trial-based cost-effectiveness analysis of bevacizumab in HER2-negative metastatic breast cancer patients: a study of the southeast Netherlands breast cancer consortium. Eur J Cancer. 2017;79:238–246.
- Dedes KJ, Matter-Walstra K, Schwenkglenks M, et al. Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer. 2009;45(8):1397–1406.
