Abstract
Introduction
Non-small cell lung cancer (NSCLC) is a leading cause of cancer mortality in the Western world. However, emerging treatment options and more patients directed to active treatments might improve the outcomes. Here, we retrospectively studied the patient characteristics and treatment practices for NSCLC in Finland 2014–2019 with a special focus on changes in trends over time.
Material and methods
The cohort consisted of patients diagnosed with NSCLC in Finland 2014–2018. Cancer treatments for the patients were followed until the end of 2019. The data, both structured and unstructured, were collected from electronic medical records of four university hospitals in Finland
Results
Of the study population (n = 4047), 65% had adenocarcinoma and 29% squamous cell carcinoma. The share of patients who had not received any active treatment (except palliative radiotherapy) decreased from 32% to 18% between 2014–18. The percentage of patients receiving surgery increased slightly from 22.7% to 24% and for patients receiving chemotherapy or immuno-oncological (IO) treatments from 29% to 41.2% and from 0.8% to 8%, respectively between, 2014–18. However, the time of treatment for patients receiving systemic cancer treatments did not change during the same time period.
Discussion
The current study suggests a trend in NSCLC towards more active treatment approaches in 2014–18.
Introduction
Lung cancer causes a significant disease burden: 12,6% of cancer incidence globally [Citation1] and 9% in the Nordic countries [Citation2] are lung cancers. During the last years, several novel drugs have been introduced for lung cancer (e.g., [Citation3–7]). The introduction of novel drugs has enabled patients with metastasised NSCLC to be more actively treated [Citation8,Citation9]. Nicolson [Citation10] estimates that novel drugs will have a significant impact on treatment time as e.g., targeted therapies and IO treatment are often continued until disease progression or intolerable toxicities.
However, it is unknown what the actual uptake of the new treatment options is, and what influence the potential increase of new treatment options might have on the use of traditional treatments and treatment combinations. It is also unknown what type of patients and cancer types are treated with what kind of treatment combinations in real life. There is furthermore little evidence on how new treatment options influence time on treatment as Nicolson [Citation10] suggests.
The aim of this descriptive study is to analyse recent (2014–2019) trends in the treatment of patients with NSCLC in Finland.
The primary objective is to describe how the treatment of patients with NSCLC in Finland has developed during the past five years. More specifically, we analyse what treatments and combinations of treatments patients with NSCLC diagnosed in 2014–2018 (followed until 2019) have received, what the characteristics of the patients in the study population are, and how the time-on-treatment has developed during the study period.
Material and methods
The study is retrospective and based on hospital register data. The study population consists of patients with NSCLC diagnosed in four out of five Finnish university hospitals: Helsinki University Hospital (HYKS), Tampere University Hospital (TAYS), Kuopio University Hospital (KYS), and Turku University Hospital (TYKS) between January 1, 2014 and December 31, 2018. The inclusion criteria are: NSCLC diagnosis (ICD-10 codes C34.X1-5) recorded for the first time between 1.1.2014 and 31.12.2018 and municipality of residence in the hospital district of the caring university hospital. Patients whose municipality of residence is outside the university hospital districts are excluded from the study. Patients with diagnoses referring to SCLC or carcinoid lung cancer (ICD-10 codes C34.X6-7) or with lung cancer diagnosis recorded prior to 2014 are also excluded from the study. The study is restricted to adult (age ≥ 18) patients at diagnosis. The final sample included 4047 patients with NSCLC. The sample includes patients that have taken part in clinical trials.
Data was gathered from hospital information systems (electronic medical records [EMR] and pharmacy information systems). Both structured and unstructured data were used. All events and episodes were linked to the patient based on a unique personal identity code. The structured EMR data includes data on inpatient days, surgical procedures, ED visits, physician and nurse visits and consultation over the phone, diagnostics, prescriptions, radiotherapy visits as well as sex, age and municipality of residence. The structured hospital pharmacy data included dates and drugs (ATC codes) for all medicines administered to each patient in the hospital.
The cancer stage and performance status of the patient at the time of diagnosis were retrieved from unstructured medical report data with keywords. Keywords for cancer stage included TNM staging classes, different cancer stages as well as words describing the stage of the tumour, such as words referring to distant metastasis. Keywords for performance status included different wordings for the ECOG, WHO, Zubrod and Karnofsky classification systems. See Appendix 1 for the list of keywords. An algorithm was used to first identify potential stage and performance status inputs and then classify the search results for the stage into categories I, II, III and IV, and for performance status into ECOG categories of 0, 1, 2, 3, and 4. The classifications were validated by a clinician and the automated classifications were validated manually line by line correcting for misinterpretations. In case the performance status input was recorded as an interval, it was interpreted as the higher number (e.g., ‘0–1′ was analysed as ‘1′). Both the stage and performance status at the time of diagnosis were identified based on the closest input date to lung cancer diagnosis date of the patient with the maximum date difference being ±60 days. For TYKS data, the stage, performance status and PD-L1 expression were retrieved manually by a clinician following the same classifications.
The treatment types analysed for all four hospitals were surgery (pneumonectomies, lobectomies and partial lung resections), radiotherapy (excluding palliative radiotherapy defined by treatment code WF004, indicating radiotherapy which aims at reducing symptoms caused by the tumour or metastases but which is not expected to affect the progression of the disease), chemotherapy (commonly used for the treatment of NSCLC), IO treatment (PD-1 and PD-L1 targeting therapies), targeted therapies (EGFR, ALK, VEGF and BRAF targeting agents), other biological treatment (defined as L01XC- and L01XE- ATC classes not included in the previous categories) and other cancer medication (defined as L01- ATC classes not included in the previous categories) (see Appendix 2). The patients were grouped into treatment combinations based on the treatment types they received (). The groups are based both on the combination of treatments the patients received. The treatment combinations are: IO treatment (defined as having received IO treatment regardless of what other treatment the patient has received), targeted therapy (defined as having received a form of targeted therapy regardless of what other treatment the patient has received, except IO), chemotherapy only, chemotherapy and surgery, surgery only, radiotherapy only, other, and palliative treatment. The “other” treatment combinations include all treatment combinations that are not included in the previous categories. For instance, if the patient has more than two treatment types (excl. IO treatment and targeted therapy), the treatment combination is categorised as “other.” “Palliative treatment” represents the treatment combination of patients for whom there were no above-mentioned treatments recorded. The group includes patients who received palliative radiotherapy, other palliative treatments or no recorded treatment at all.
Table 1. Individual treatment types and combinations were considered in the study.
Total treatment time was calculated for all patients as the difference between the dates of the patient’s first treatment visit in the first modality and last treatment visit of the last modality or treatment line, rounded down to the closest full month of treatment. For patients receiving oral medicines, total treatment time was defined as the date of the first prescription in the hospital records and the date of the last prescription. Total treatment time was calculated for patients diagnosed in 2014–2017, because for patients diagnosed in 2018, the time frame was too short to determine reliably who are still in treatment and whose treatments have ended.
The Mann-Kendall test [Citation11,Citation12] was used to detect statistically significant treatment trends. In this test, the null hypothesis (H0) is that there is no increase or decrease trend in patient numbers for some treatment over time. The alternate hypothesis (H1) is that a trend exists and that the number of patients increases or decreases over time. The Mann-Kendall test was implemented using the Python package 'mkt' [Citation13].
Research permits were obtained from all the hospitals, that provided data. According to Finnish legislation, no ethical approval is needed for retrospective register studies.
Results
Patient demographics
The annual incidence of lung cancer in Finland is approximately 2700, out of which 75% is estimated to be NCLC [Citation14], and thus the total number of new NSCLC patients diagnosed in Finland during 2014–2018 is estimated to be approximately 10–100. The total population in the study is 4047 patients (). Of the total study population, 51% were treated in HYKS, 20% in TYKS, 21% in TAYS, and 8% in KYS. Out of the study population, 65% were diagnosed with adenocarcinoma, and 29% with squamous cell carcinoma. For the patients for whom data on the stage of the disease could be found in the patient records at the time of diagnosis (28% of the patients, n = 1133); 49% were identified with stage IV, 22% stage III, 12% stage II, and 17% stage I. The performance status was identified for 30% of the population (n = 1195). Amongst these, ECOG 1 represented the largest share with 47%, followed by 21% for ECOG 0 and 20% for ECOG 2. In addition, PD-L1 expression was identified for 16% of the population (n = 663). Numerous tests for PD-L1 expression were used and the PD-L1 50% expression threshold was used regardless of the assay platform. Out of patients for whom PD-L1 expression could be identified, 31% had strong positive expression in tumour cells (expression 50% or higher), 32% had weak positive expression (expression 1–49%), 3% had positive but undefined expression and 35% negative expression (expression less than 1%).
Table 2. Patient demographics (diagnosis, stage, performance status, PD-L1 expression, age, and sex).
There is an increase in the number of patients whose performance status was recorded. For patients diagnosed in 2014, the performance status could be identified for 22% of the patients. For patients diagnosed in 2018 performance status could be identified for 42%. An identifiable recording of the stage at the time of diagnosis inpatient records could be found for 19% of the patients diagnosed in 2014 and equivalently for 42% of patients diagnosed in 2018. No significant change in the distributions of performance status or stage could be identified. Out of the four university hospitals in the study, Turku University Hospital (TYKS) had the highest rate of staging (67%). While the overall rate of staging in the population is relatively low (28%), the distribution of stages in the combined data is similar to the distribution in the TYKS data.
Individual treatment types
To understand how individual treatment types have changed for patients diagnosed with NSCLC 2014–2018 and treated 2014–2019, we analysed the trend of the share of patients who had received particular treatment regardless of other treatments they may have received. In total, 3109 patients received some kind of active treatment. Radiotherapy (only, or in combination with other treatment types) was most common (): 42.5% of the patients in our cohort received radiotherapy. The trend for treating NSCLC with radiotherapy is fairly stable, with no significant increase or decrease, fluctuating between 36.2% of the patients diagnosed in 2014 to 40.8% of the patients diagnosed in 2018. Out of all patients in our study, 36.9% received chemotherapy specific for lung cancer (only, or in combination with other treatment types). The use of chemotherapy specific for lung cancer shows a significant (p = 0.049) increase from 29% of the patients diagnosed in 2014 to 41.2% for patients diagnosed in 2018.
Table 3. Patients per individual treatment types by year of diagnosis.
The number of patients who received surgery (only, or in combination with other types) has also increased significantly (p = 0.017). Patients who received surgery constituted 22.7% of the patients diagnosed in 2014 and 24.0% of the patients diagnosed in 2018. IO treatments show an increase from 1% of patients diagnosed in 2014 to 8% of patients diagnosed in 2018. The other individual treatment types that were less common show no significant increase or decrease.
Treatment combinations
To further understand comprehensive treatment trends, we grouped the individual treatment types into treatment combinations. The distribution of patients grouped into the different treatment combinations (group of treatments or individual treatment see ) depending on the year of diagnosis is presented in . Patients diagnosed in 2014–2018 and their treatments in 2014–2019 are included in the analysis. As can be seen from the figure, there is an increase in the proportion of patients receiving only surgery from 12% in 2014 to 15% in 2018, which is statistically significant (p = 0.008).
Figure 1. Treatment combinations trend: Number of patients in each treatment combination category by year of diagnosis 2014–2018.
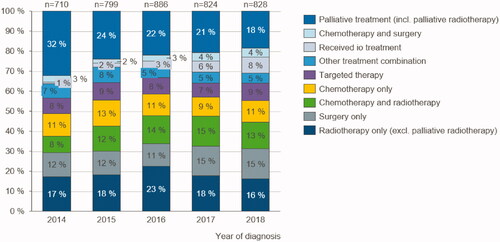
The percentage of patients who have received targeted therapies and more complex treatment combinations (labelled as “other treatment combinations”) has remained fairly stable. The share of patients receiving IO treatments increases from 1% of patients diagnosed in 2014 to 3% of patients diagnosed in 2016 and 8% for 2018. The increase of patients treated with IO is statistically significant (p = 0.001).
The patients who have not received any active treatment are defined in our analysis as patients who have received palliative treatment only (including palliative radiotherapy). The share of these patients has decreased significantly (p = 0.009) from 32% of the patients diagnosed in 2014 to 18% of patients diagnosed in 2018.
Impact of patient profiles on treatment trends
The main patient characteristics we studied are the stage of the disease and the performance status of patients at the time of diagnosis.
Patients with a performance status of 0 or 1 close to the time of diagnosis received a wide variety of treatments (). Almost all (96%) patients diagnosed 2014–2018 with a status of 0 received some type of active treatment. The same is true for patients with performance status 1 (92% received active treatments). However, out of patients with a performance status of 3 or 4, less than 50% received any type of active treatment, and radiotherapy only was the most common treatment combination in these groups.
Figure 2. Lung cancer patients’ performance status in relation to treatment combinations: Number of patients in each treatment combination category by performance status and stage of lung cancer at the time of diagnosis 2014–2018.
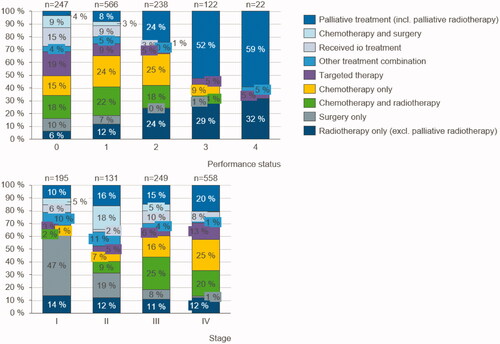
also shows the treatment combinations with respect to the identified stage of cancer. Almost half (47%) of the patients with stage I lung cancer received “surgery only,” as did 19% of the patients with stage II cancer. The patients who were diagnosed with stage IV cancer were treated with chemotherapy as well as with chemotherapy in combination with radiotherapy. One-fifth (20%) of patients with stage IV lung cancer at the time of diagnosis did not receive any active treatment.
Trend in pharmacological treatment
When studying the number of individual systemic cancer treatments (defined as distinct ATC codes) received by patients, who received some form of systemic treatment, no significant increase in the average number of different medications per patient can be observed: patients diagnosed in 2014 had on average of 2.3 different medications while patients diagnosed in 2017 had 2.5.
The total time of treatment has remained roughly the same for patients receiving some form of systemic treatment and diagnosed during the years 2014–2017. Although more patients diagnosed in 2016 lived past two years than those diagnosed in 2014 and 2015, the difference in treatment time is non-significant and the change doesn’t hold for those diagnosed in 2017.
Discussion
This study describes the developments in the NSCLC patient population and the treatments they received in Finland in 2014–2019. We also studied the distribution of treatment combinations depending on the stage and performance status of the patients. The basic characteristics of the patient population (age, sex, type of cancer, stage and performance status) did not change during the time period.
A change in the trend of treatment combinations could be detected. The increase of the treatment “surgery only” supports research results [Citation15] of a trend towards an increased number of operations. The increase can be due to an increase of computed tomography diagnostics and thus detecting earlier stages of tumours and also rather indolent adenocarcinomas often presenting radiologically as ground-glass opacities. However, for this study population as a whole, there seems to be no significant increase in the share of patients diagnosed in an earlier stage. The result of an increase of surgery as a treatment is also in line with findings by e.g., Driessen et al. [Citation16] suggesting a steady increase in the proportion of patients undergoing surgery in the Netherlands between 1990 and 2014, as well as with findings by Møller et al. [Citation15] showing an up to 17% share of patients receiving surgery in England – in our study the proportion of patients undergoing surgery (only, or in combination with other treatment types) was 22.8%.
Although a trend towards personalised medicine and an increase in the use of targeted therapies and IO treatment is described in the literature [Citation17], we could not find an increasing trend in the use of targeted therapies for patients diagnosed in Finland during 2014 and 2018 and treated 2014–2019. However, the use of IO treatments shows increase, reflecting both clinical trial activity during those years as well as the ensuing more widely adopted use of the new drugs in routine practices.
There is a significant decrease in the ‘palliative treatment’ category during the observation period meaning that more and more patients with NSCLC receive some kind of active treatment even though there is no significant change in the stage or performance status of the patients over the observation period. This indicates that treatments have become more available especially for patients with stage III or IV cancer since these were the patients most often receiving only palliative treatments.
When interpreting the changes in the prevalence of treatment combinations and the average number of different medications received by the patients, one needs to take into consideration that the follow-up periods are of different lengths: follow-up for patients diagnosed in 2018 is only 1–2 years whereas for patients diagnosed in 2014 the follow-up can be up to 5–6 years if the patients have survived that long. This affects especially the prevalence of treatments that are more common in later stages of the disease or in later treatment lines. Thus, for example, the increase in the prevalence of IO treatments can in reality be higher, than what is now observed, since some of the patients diagnosed in 2018 may receive IO treatments after 2019. This bias, however, should not affect the prevalence of surgeries, since surgery is typically a first line of treatment initiated soon after the diagnosis. Also, the prevalence of palliative treatment is only comparable across cohorts, since if patients are to receive active oncological treatments, at least one of them would most likely be given during the first year following the diagnosis.
The emergence of IO-treatments and the ensuing increase in them and the overall increase of active treatment, seem not to, as of yet, result in significantly longer treatment periods. This is not in line with the prediction made by Nicolson [Citation11]. One reason could be that the share of patients receiving IO treatments is still rather small. Also, simultaneously the share of patients receiving some kind of active treatment increases significantly. It is possible that the total treatment time for the kind of patients, who previously received only palliative care, are shorter than average, which would offset the increased total treatment times for other patients.
A clear strength of the study is that the data is extracted from the university hospitals’ electronic medical records, and represents real-world data. Also, the data is representative of the whole country, since even though the university hospitals cover only 53% of the population, it is their responsibility to coordinate care practices with the central hospitals in their respective regions providing care for the rest of the population. Hospital records in Finland are perceived as very reliable. The accuracy of the disease diagnoses and the registration coverage has been proven to be good as more than 95% of discharges can be identified from the national registers [Citation18]. Also, structured data related to treatments is highly reliable since it forms the basis for billing. However, unstructured data is not as uniformly recorded and its coverage is not as good, as can be seen with stage and performance status data.
There are some limitations to the study. There are different recording practises in each hospital concerning unstructured data. The unstructured data extracted from patient records has been manually validated with clinicians. Due to the different recording practises (and terminology used) and the need for clinical interpretation of the unstructured data, some tumour descriptions could not be interpreted, and stage IV may be overly represented as it can be easily identified from its natural language description (e.g., distant metastasis). The future analysis would benefit from performance status and the stage being recorded as structured data. Furthermore, comorbidities for each patient were not taken into account. Thus, patients with other diagnoses (than NSCLC diagnosis) are not stratified in the data. Lastly, time-dependent trends may be difficult to detect in a 5-year time span and future studies would benefit from additional years.
Based on the study results, there is an initial indication of a shift in the treatment of patients with NSCLC. It would be interesting in the future, when data for additional years become available, to analyse and verify whether the identified shift in treatment indicated a long-term trend. Also, investigating the changes in the number of treatment lines would be interesting in the future. This analysis, however, would benefit from a better coverage of stage information than what was available in this study. An international comparison could as well be conducted. Also, changes in survival should be considered, once long enough a time period is available and all relevant factors can be stratified. Lastly, the impact of the changes in treatment trends on the total cost of care should be studied.
Conclusion
The aim of this descriptive study was to analyse recent (2014–2019) trends in the treatment of patients with NSCLC in Finland. In conclusion, new treatment options such as IO-treatments have not replaced traditional treatment types, but are rather used as an addition to already existing treatment types and combinations. The results also indicate that patients are treated overall more than before, and the share of patients receiving only palliative care is decreasing. The overall trend of more active treatment seems not to be due to changes in patient or cancer characteristics, since they did not show significant change over the study period.
Data availability statement for “Trends in treatment of Non-Small Cell Lung Cancer in Finland 2014–2019.”
Raw data were generated at University Hospitals in Finland. Derived data supporting the findings of this study are available from the Finnish university hospitals: Helsinki University Hospital (HYKS), Tampere University Hospital (TAYS), Kuopio University Hospital (KYS), and Turku University Hospital (TYKS) upon request.
Disclosure statement
R-L.L., E.P., I.H., F.H. and S.K. reports that their employer has received funding from Merck and Co., Inc. K.N., S.K., A-M.A. were employees of Merck and Company, Inc. at the time of the study. S.T., M.S., J.A., A.K., and J.K. reports having received consultancy fees from Merck and Co., Inc.
References
- Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide, version 2.0, IARC CancerBase. 2004. No. 5. Lyon: IARC Press.
- Danckert B, Ferlay J, Engholm G, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries. Assoc Nord Canc Registries Dan Canc Soc. 2019.
- Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis. 2018;10(Suppl 13):S1447–S1460.
- Hirsch F, Scagliotti G, Mulshine J, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311.
- Leidl R, Wacker M, Schwarzkopf L. Better understanding of the health care costs of lung cancer and the implications. Expert Rev Respir Med. 2016;10(4):373–375.
- Koivunen J, Knuuttila A, Mali P. Levinneen keuhkosyövän nykyaikainen lääkehoito – mitä totunnaisten solunsalpaajien lisäksi? Duodecim. 2016;132:555–560.
- Palmer S, Kuhlmann G, Pothier K. IO nation: the rise of immuno-oncology. CPPM. 2015;12(3):176–181.
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340.
- Goldberg S, Gettinger S, Mahajan A, et al. Durability of brain metastasis response and overall survival in patients with non-small cell lung cancer (NSCLC) treated with pembrolizumab. J Clin Oncol. 2018;36(15_suppl):2009–2009.
- Nicolson M. Lung cancer survival: progress and challenges. J Thoracic Oncol. 2019;14(10):S24.
- Mann H. Non-parametric test against trend. Econometrica. 1945;13(3):245–249.
- Kendall M. Rank correlation methods. fourth ed., London: Charles Griffin; 1975.
- Goswami B. Python module to compute the Mann-Kendall test for trend in time series data. GitHub Repository. 2017.
- Duodecim. Keuhkosyöpä. [cited 2021 September 13] Available from: https://www.terveyskirjasto.fi/dlk00031.
- Møller H, Coupland V, Tataru D, et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax. 2018;73(6):530–537.
- Driessen E, Aarts M, Bootsma G, et al. Trends in treatment and relative survival among non-small cell lung cancer patients in The Netherlands (1990–2014): disparities between younger and older patients. Lung Cancer. 2017;108:198–204.
- Zappa C, Mousa M. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300.
- Järvelin J, Ryynänen H, Mahkonen H, et al. Kohti luotettavaa syövän hoitoon pääsyn seurantaa: Hilmon ja syöpärekisterin diagnoositietojen vertailu [towards reliable monitoring of access to cancer treatment: comparison of the diagnosis data of the national hospital discharge register and the finnish cancer registry]. Finnish Medical Journal. 2019;45(74):2581–2588.
