Background
Hemophagocytic lymphohistiocytosis (HLH) is a rare and severe systemic immune activation syndrome that may drive multiple organ failure requiring advanced life support in the ICU [Citation1]. The diagnosis of HLH in critically ill patients is presumptive, based on nonspecific variables combined in the HLH-2004 criteria or in the H-score [Citation2]. Hematological malignancies, at the time of diagnosis or relapse, account for leading underlying conditions in adult-acquired HLH [Citation3,Citation4]. The treatment of hematological malignancy-related HLH relies on immunomodulatory prephase including etoposide and corticosteroids, associated with disease-adapted combination chemotherapy [Citation4,Citation5]. Achieving a fast control of unleashed immune activation is paramount to improve the prognosis of HLH. Observational studies have suggested that early etoposide initiation may improve the prognosis of malignancy-related HLH [Citation6–8]. We herein addressed the effects of etoposide-containing regimens on HLH-defining criteria and organ failures in critically ill patients with hematological malignancy-related HLH.
Material and methods
This retrospective study was carried out between 2013 and 2020 in a medical ICU within a tertiary care center comprising a department of clinical hematology. Adult patients who received etoposide in the ICU were identified from the pharmacy registry. The diagnosis of HLH was deemed probable by fulfillment of five HLH-2004 criteria or by high probability provided by the H-score. The underlying hematological malignancy was definitely proven by histological or cytological diagnosis. Dose reduction of etoposide was considered owing to organ dysfunctions [Citation4,Citation5]. Body temperature was monitored with an ear thermometer. Organ failures were assessed daily by a modified non-platelet Sequential Organ Failure Assessment score (npSOFA, ranging from 0 to 20) taking into account circulatory, ventilatory, renal, neurological, and hepatic failures, but devoid of its platelet component likely impaired by bone marrow infiltration, cytostatic compounds, and platelet transfusions. The one-year follow-up was available for all patients. The study was approved by the ethics committee of the French Intensive Care Society (#CE-SRLF-17-03). Three patients were already included in a previous report [Citation9]. Continuous variables were expressed as median (interquartile range) and categorical variables as counts (percentages) and compared using paired Wilcoxon test and Fisher test, respectively.
Results
Twenty-four patients were included in the study (). Sixteen patients fulfilled at least five HLH-2004 diagnostic criteria. The H-score was computed at high values of 253 [226.3–287.0], corresponding to HLH probability higher than 99% [98–>99]. Eight patients had less than five HLH-2004 criteria but with a median H score of 226 [178.3–243.5] resulting in a HLH probability of 98%. Figures of hemophagocytosis were found in 13 patients (56.5%). Hematological malignancies were generally related to mature T and B lymphoma (n = 17, 70.8%), and were often recently diagnosed (n = 20, 83.3%). Supports for organ failures were frequently initiated, including invasive mechanical ventilation (n = 13, 54.2%), vasopressors (n = 17, 70.8%), or renal replacement therapy (n = 13, 54.2%). Etoposide (median dose of 97.7 mg/m2 [86.5–105.5] per day for one or two injections) was administered early (median 0.5 d [0–2.25]) after the diagnosis of HLH was set, and was combined with high-dose steroids in 21 patients (87.5%). Four patients died rapidly before receiving additional chemotherapy. Disease-directed chemotherapy was initiated in 16 patients (58.3%) within one month following ICU admission (median of 4 d [1.5–7.0] from etoposide) and was initiated after one month in four patients.
Table 1 Clinical characteristics of patients before treatment initiation.
After etoposide administration, the body temperature decreased from 38.9 [38.0–39.5] to 37.6 °C [36.8–39.0] (p = .01) within 24 h (). The ferritin level decreased by 59.8 ± 28.9% within 7 d, from 24731 [11906–72318] to 9313 µg/L [3007–13688] (p < .0001) (Supplementary Figure 1(A)). Other potential markers of HLH activity were also modulated within 7 d from etoposide, including lactate dehydrogenase levels that decreased from 2121 [837.5–3402.5] to 636 UI/L [481–1394] (p = .03) and prothrombin ratio that increased from 61 [51–73] to 82% [65–86] (p < .0001) (Supplementary Figure 1(B,C)). Triglycerides and fibrinogen levels were most often normal at the time of HLH diagnosis, precluding significant variations over time. Leukocyte count significantly decreased from 4980/mm3 [1625–9670] at the time of etoposide initiation to 250/mm3 [160–1075] at day 7 (p = .0002), likely related to drug-induced myelosuppression. Hemoglobin and platelet counts did not change significantly over the first 7 d from etoposide, albeit biased by transfusion of blood products.
Figure 1. Time course of body temperature daily peak (A), lactate level (B), and non-platelet sequential organ failure assessment (npSOFA) (C) during the first week after etoposide injection (day 0). *p < .05.
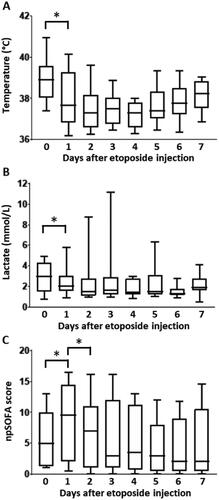
Following etoposide treatment, lactate level decreased from 3.0 [1.5–4.3] to 2.1 mmol/L [1.6–3.0] in 24 h (p = .02) (). With regard to organ failures, patients exhibited early and transient deterioration in npSOFA that significantly increased from 5 [1.3–10] at etoposide initiation to 9.5 [2–15] at day 1, and thereafter improved to npSOFA value of 7 [1–11] at day 2 (). The in-ICU mortality rate was 45.8%, including four patients who died from multiple organ failure related to the underlying malignancy, and six patients who died later on from secondarily acquired sepsis. Hospital and one-year mortality were 54.2 and 66.7%, respectively.
Discussion
Time course of organ failures over the first days of ICU management is a major prognostic factor in critically ill patients with malignancies [Citation10]. Along this line, the prognosis of HLH relies on early awareness of the disorder as well as prompt diagnostic work-up to investigate the underlying condition and initiate appropriate treatment. However, obtaining an accurate diagnosis of hematological malignancy may require a few days, while under the threat of fast clinical deterioration to multiple organ failure. In this setting, immunomodulatory treatment is aimed at rapidly tune down the unleashed immune activation syndrome responsible for severe clinical alteration or overt organ failures [Citation2]. Etoposide combined with steroids has thus emerged as the preferential first-line treatment of severe malignancy-associated HLH, eventually followed by disease-adapted combination therapy. This sequential strategy echoes with the concept of prephase (or progressive) chemotherapy to avoid dramatic tumor lysis syndrome and further deterioration of organ dysfunction, as dreaded in Burkitt lymphoma or hyperleukocytic leukemia. As a matter of fact, early prephase etoposide treatment, commonly used in combination with corticosteroids, was associated with relatively good outcomes since about one half of patients survived the current hospitalization and one-third remained alive at one year. This is consistent with the one-year survival rate of 40% recently reported in patients with lymphoma-related HLH initially treated with etoposide-containing regimen [Citation8].
The results reported here are consistent with a potent activity of etoposide onto the pathophysiological processes of HLH. Temperature and ferritin levels were highly reactive toward etoposide, most often combined with corticosteroids. Although very unspecific and therefore not included in diagnostic criteria, lactate dehydrogenase levels rapidly decreased as well after etoposide. In contrast, other variables appeared not relevant to address the efficacy of etoposide. Assessment of cytopenia was likely confounded by the myelotoxicity of etoposide and other cytostatic drugs and the transfusion of blood products. Most importantly, etoposide-containing regimens were associated with decreased lactate levels and subsequent improvement in organ failures, despite initial transient worsening in npSOFA possibly related to tumor lysis syndrome and cytokine release [Citation1]. Only four patients actually died from multiple organ failure ascribed to HLH and/or to the underlying malignancy.
Etoposide works through inhibition of topo-isomerase 2 enzyme and is primarily viewed as a cytostatic chemotherapeutic agent [Citation11]. It is frequently used to this aim for the treatment of various hematological and solid neoplasms. Besides, etoposide may also harbor alternative immunomodulatory properties relevant for the treatment of HLH. In an experimental model of HLH, etoposide led to efficient suppression of the inflammatory cytokine interferon-γ and was able to rescue perforin-deficient mice infected with lymphocytic chorio-meningitis virus (LCMV). Interestingly, etoposide did not drive direct anti-inflammatory effect on macrophages or dendritic cells, but selectively eliminated activated T cells [Citation12]. Alternative treatments including ruxolitinib, intravenous immunoglobulins or cytokine inhibitors (e.g., tocilizumab and anakinra) have been proposed to treat HLH, but their added value in terms of efficacy and delay of action are unclear [Citation13,Citation14].
In conclusion, this observational study highlights the potent activity of etoposide on the immune activation syndrome of hematological malignancy-related HLH. Etoposide-containing regimen was able to rapidly dampen some dynamic HLH-defining variables and to eventually improve organ failures.
Supplemental Material
Download MS Word (11.5 KB)Supplemental Material
Download TIFF Image (132 KB)Disclosure statement
FP: ALEXION PHARMA (institutional grant) and GILEAD SCIENCES (consulting and teaching personal fees).
Data availability statement
The data that support the findings of this study are available from the corresponding author, FP, upon reasonable request.
References
- Buyse S, Teixeira L, Galicier L, et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–1702.
- Valade S, Monseau G, Mariotte E, et al. Diagnostic performance of hemophagocytic lymphohistiocytosis criteria and HScore in critically ill patients with severe hemophagocytic syndrome. Crit Care Med. 2021;49:e874–e879.
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516.
- Setiadi A, Zoref-Lorenz A, Lee CY, et al. Malignancy-associated haemophagocytic lymphohistiocytosis. Lancet Haematol. 2022;S2352302621003665.
- La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477.
- Arca M, Fardet L, Galicier L, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168(1):63–68.
- Knaak C, Schuster FS, Nyvlt P, et al. Treatment and mortality of hemophagocytic lymphohistiocytosis in adult critically ill patients: a systematic review with pooled analysis. Crit Care Med. 2020;48(11):e1137–e1146.
- Song Y, Wang J, Wang Y, et al. Requirement for containing etoposide in the initial treatment of lymphoma associated hemophagocytic lymphohistiocytosis. Cancer Biol Ther. 2021;22(10–12):598–606.
- Cherruault M, Le Goff M, Tamburini J, et al. Urgent chemotherapy in Sepsis-Like shock related to hematologic malignancies. Crit Care Med. 2018;46(5):e465–e468.
- Lecuyer L, Chevret S, Thiery G, et al. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–814.
- Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34(10):1514–1521.
- Johnson TS, Terrell CE, Millen SH, et al. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91.
- Dufranc E, Del Bello A, Belliere J, et al. IL6-R blocking with tocilizumab in critically ill patients with hemophagocytic syndrome. Crit Care. 2020;24(1):166.
- Wohlfarth P, Agis H, Gualdoni GA, et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2019;34(9):723–731.
