Abstract
Background
Chemoradiotherapy (CRT) may induce myocardial dysfunction, congestive heart failure, and impaired physical performance in patients with esophageal cancer (EC). We aimed to investigate left ventricular (LV) function at rest and during stress, using echocardiography (echo) and a cardiopulmonary exercise (CPX) test both before and immediately after completing CRT.
Material and methods
Consecutive EC patients referred for curative treatment were enrolled. Patients attended either definitive CRT or neoadjuvant CRT with subsequent surgery. The evaluation included cardiac biomarkers, electrocardiogram, echo, and CPX test. The primary endpoint was changes in left ventricular (LV) global longitudinal strain (GLS) at rest. Secondary endpoints were LV ejection fraction (LVEF), LV diastolic function, LVEF and GLS at peak exercise, and maximal oxygen consumption (VO2max). The trial was registered with ClinicalTrials.gov (NCT03619317).
Results
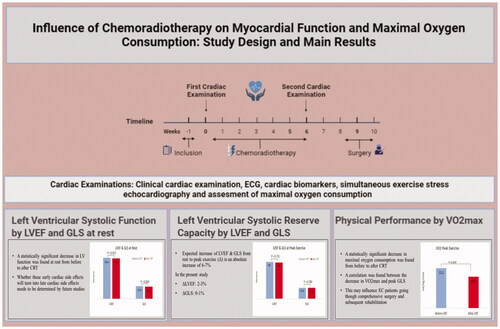
Among 47 patients enrolled (94% male; median age 67 years, range 50–86 years), cardiac examinations were performed a median of three days [Interquartile range (IQR (1–5))] before CRT and one day [IQR (0–6)] after CRT. At rest, GLS and LVEF decreased, 17.6 vs. 16.4% and 56.4 vs. 55.1%, respectively (p = 0.004; p = 0.030). Furthermore, an absolute decrease of at least 5% in LVEF and 2.5% in GLS was noted in 21% of the patients. Signs of LV diastolic dysfunction increased from 13 to 21% (p = ns). VO2max significantly decreased; 21.2 ml/kg/min vs. 18.8 ml/kg/min (p < 0.001).
Conclusion
LV function and physical performance decreased in EC patients after CRT, and the LV systolic reserve capacity declined. This study highlighted that EC treatment was associated with early cardiac side effects, which may have clinical and prognostic implications.
Graphical Abstract
Introduction
Chemoradiotherapy (CRT) alone or followed by surgery improves survival in patients with esophageal or gastroesophageal junction cancer (EC) [Citation1]. However, concerns have been raised over the potential cardiotoxicity of CRT treatment as CRT may induce myocardial dysfunction, including clinical heart failure, arrhythmia, and subsequently reduced physical performance [Citation2–5]. Data from a Swedish study have shown that neoadjuvant chemoradiotherapy (nCRT) induces an acute impairment of left ventricular (LV) diastolic dysfunction and LV systolic function with a decrease in the mitral annular plane systolic excursion (MAPSE) [Citation4]. The global longitudinal systolic strain (GLS) has been shown to provide additional information about LV function beyond LVEF [Citation6]. Furthermore, GLS has shown to be superior to the LVEF in detecting myocardial dysfunction following the cardiotoxic treatment seen in cancer patients [Citation7,Citation8]. The evaluation of LV function during physical stress contributes additional information about the LV systolic reserve capacity and may serve as a sensitive marker of subtle changes of systolic myocardial function [Citation9,Citation10]. The cardiopulmonary exercise capacity provides independent prognostic information when evaluating the patients’ daily living activity [Citation11]. Data are sparse on alterations of LV function at rest and during exercise and their association with exercise capacity in EC cancer patients following a CRT treatment course. Therefore, the present prospective study aimed to (a) determine the LV function at rest and peak exercise by advanced echocardiography (echo) before and immediately after CRT, (b) to examine the potential alterations in peak cardiopulmonary exercise capacity by oxygen consumption assessment, and (c) the relation of changes in oxygen consumption to LV function changes during and after the CRT treatment course.
Material and methods
Patient inclusion
Between June 2018 and February 2021, a total of 59 consecutive patients with primary EC cancer were identified at the Department of Oncology, Aarhus University Hospital, Denmark. Patients were prospectively enrolled after providing informed and written consent according to the principles of the Helsinki Declaration. Four patients were excluded before the baseline examination. An additional eight patients were excluded before the follow-up examination as presented in a CONSORT diagram in the Supplementary Section, yielding a study population of 47 patients [Citation5]. The inclusion criteria were 18 years of age or more; a World Health Organization (WHO) performance status (PS) of 0, 1, or 2; histologically verified locally advanced non-metastatic squamous cell carcinoma or adenocarcinoma referred for definitive chemoradiotherapy (dCRT) or nCRT followed by surgery [Citation1,Citation12].
The patients underwent two cardiac examinations at the Department of Cardiology, Aarhus University Hospital, Denmark. The baseline clinical cardiac examination was performed before dCRT or nCRT; the second cardiac examination (follow-up (F-U)) was performed within a week after CRT had been completed. The cardiac examinations comprised assessment of cardiac history; physical examination including measurement of weight, height, and biochemical testing; 12-lead electrocardiogram; comprehensive transthoracic echo (TTE) at rest and during exercise; and a symptom-limited, semi-supine cardiopulmonary exercise (CPX) test with an assessment of peak oxygen consumption (VO2max). An automatic blood pressure monitor was used to assess blood pressure during quiet resting conditions over 20 min. New York Heart Association functional classification (NYHA), Charlson Comorbidity Index (CCI), and Clinical Frailty Score were evaluated [Citation13–15].
The primary endpoint was changes in GLS at rest. The secondary endpoints were LV ejection fraction (LVEF), LV diastolic function, LVEF and GLS at peak exercise, and VO2max. All endpoints were measured before CRT had been initiated and after CRT had been completed. Changes of GLS and LVEF in absolute values of 2.5 and 5%, respectively, were considered clinically significant both at rest and at peak exercise before and after CRT [Citation16,Citation17]. Diastolic function was classified as normal or characterized as one of three severity grades [Citation18]. In the present study, myocardial dysfunction was defined as present if either one of the parameters 1–4 or two of the parameters 1–6 were met: (1) LVEF ≤ 50%; (2) GLS < 18%; (3) N-terminal pro b-type natriuretic peptide (NT-ProBNP) ≥ 300 ng/l; (4) LV diastolic dysfunction; (5) Delta (Δ)-LVEF peak stress-rest < 5%; (6) Δ-GLS stress-rest < 2.5%.
The study was approved by the Danish Data Protection Agency and the local scientific, ethical committee of the Central Denmark Region. Furthermore, the trial was registered with ClinicalTrials.gov (NCT03619317) before study initiation.
Cancer treatment
Cancer treatment consisted of weekly intravenous (iv) chemotherapy with paclitaxel 50 mg/m2 and carboplatin determined by the area under the curve (AUC) 2 and concomitant radiotherapy (RT). RT was delivered following Danish Esophageal Cancer (DECV) 2018 guidelines. Target definition of tumor (GTVt) plus locoregional lymph nodes (GTVn) were defined malignant based on a diagnostic f-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT), endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS), magnetic resonance imaging (MRI), and pathology or a mix of these diagnostic modalities. A planning four-dimensional (4D) PET/CT scan was used to monitor and adapt target movements. The GTVs were expanded to CTV (clinical target volume), respiratory motion was included (iCTV), and finally expanded to the planning target volume (PTV), including all other treatment and delivery-related uncertainties. The PTV margins reflected treatment using daily cone-beam soft tissue match and a strict adaptive strategy [Citation19]. Most patients (n = 46) were treated using 5–8 6 MV intensity-modulated radiation therapy (IMRT) fields. Non-coplanar fields were used when necessary to avoid direct fields through the heart. One patient was treated with pencil beam scanning proton therapy. The dCRT group received 50 Gy in 25 fractions (n = 3), or 50 Gy in 27 fractions (n = 6) over six weeks. The nCRT group received 41.4 Gy in 23 fractions (n = 36) over five weeks, 50 Gy in 25 fractions (n = 1), or 50.4 Gy in 28 fractions (n = 1) over six weeks followed by surgery no earlier than 3–4 weeks after completing nCRT [Citation1]. Photon plans were made covering the PTV with a homogeneous dose (95–107%). The heart’s dose constraints were V40Gy < 30% (30% of the heart was kept below 40 Gy) and V25Gy < 50%. Lung mean dose (MLD) < 20 Gy, V20Gy < 35% and V5Gy max 60%. Delineation of the heart followed Danish national guidelines for lung (DOLG) and breast (DBCG) cancer.
Blood samples
Venous blood samples were collected on the day of the baseline examination before treatment initiation and when CRT was completed on the day of F-U to analyze Troponin T (TNT) (<14 ng/l) and NT-ProBNP (<300 ng/l), cholesterols, triglyceride, hemoglobin, leucocytes, and creatinine.
Echocardiography
A comprehensive TTE was performed according to the current guidelines issued by the European Society of Cardiology. TTE was performed at the Department of Cardiology, Aarhus University Hospital. TTEs and all analyses were performed by a single investigator (M.M.A.S.) [Citation18,Citation20]. The ultrasound system used was (Vivid E95; GE Healthcare, Horton, Norway). The investigator was blinded to all the clinical data.
LV systolic function was evaluated by GLS and LVEF at rest and during exercise. GLS was assessed by standard two-dimensional (2D) images with a frame rate of ≥55 frames per second for speckle tracking analysis [Citation21]. LVEF was assessed by three-dimensional (3D) images. In patients with poor image quality during exercise, 2D images were used for LVEF assessment and calculated by the Simpson biplane methods of disks. LV diastolic function was evaluated according to current guidelines by the European Society of Cardiology [Citation18]. RV systolic function was evaluated by M-mode measurement of tricuspid annular plane systolic excursion (TAPSE) and RV systolic longitudinal function (RV GLS).
Cardiopulmonary exercise protocol
To investigate EC patients’ physical performance, a cardiopulmonary exercise test was performed with the patients in semi-supine position with lateral tilt assessing the VO2max. A symptom-limited multistage max test using the Echo Cardiac Stress Table (Lode, Groningen, the Netherlands) [Citation22] with workload starting at 0 Watt (W) and a stepwise 25-W increase every 3 min was used. At each exercise stage, echocardiographic images of LVEF and GLS were obtained.
Patients were encouraged to exercise until exhaustion (Borg > 18) with a fixed pedaling speed of 60 rounds per minute. The target respiratory exchange ratio (RER) was ≥1.1, and the metabolic equivalent of task (METs) was used to show the rate of intensity. Patients were monitored by pulse oximetry, continuous 12-lead electrocardiogram, and blood pressure measurements.
Statistics
Patient characteristics following approximate normal distributions are expressed as mean ± standard derivation (SD) or median with interquartile range [IQR (Q1–Q3)] when values were continuous; frequencies and corresponding percentage when values were categorical. Data were compared using the two-sample t-test or the Wilcoxon rank-sum test depending on normality, Pearson’s chi-squared test, or Fisher’s exact test. A Q–Q plot and histograms were used to check continued values for normality. Patient, echocardiographic, and CPX measures before and after CRT were compared using paired t-test when values were continuous and normally distributed and by the Sign rank test for categorical- or non-normally distributed continuous values. Correlation analyses were performed by Pearsons or Spearman correlation tests. All statistical analyses were performed using Stata/IC version 15.1 (StataCorp LLC, College Station, TX, USA). p-Values below 0.05 were considered statistically significant.
The study was designed to include 56 patients based on a power calculation with an expected 20% patient dropout where a change in peak GLS of 2.5% (absolute value) is considered clinically significant. To achieve a statistical power of 90%, the study needed a sample size of 46 patients, given a standard deviation of 2% and a two-sided alpha value of 0.05.
Results
Patient characteristics
Baseline characteristics of the 47 Caucasian EC patients completing the study are shown in . The median age of the participating patients was 67 years; 94% were males. Tumors were as follows: GEJ 74%; adenocarcinomas 74%. TNM classification consisted of 89% ≥ T3 and 51% ≥ N1. A total of 36 patients were referred to nCRT. In all, 55% of the patients had hypertension, ischemic heart disease (IHD), or known arrhythmia when commencing cancer treatment. The median time from baseline examination to CRT initiation was three days [IQR (1–6)]; from CRT to F-U, one day [IQR (0–6)]. The median number of weekly chemotherapy courses was five [IQR (5–5)]. All patients completed the prescribed radiation dose. The median GTVt volume was 36.2 cm3 (range 2.6–174.0), while for the 29 patients with malignant lymph nodes, the median GTVn volume was 3.7 cm3 (range 0.3–22.8). ICTV and PTV volumes were in median 233 cm3 (range 527–42) and 580 cm3 (range 215–1051), respectively. Large variability in radiation dose to both lungs and heart was observed. The mean dose to the lungs varied between 1.1 and 13.9 Gy with a median of 8.3 Gy, while the mean dose to the heart varied from 0.6 to 24.7 Gy with a median of 12.61 Gy. Similarly, V25Gy and V40Gy were median 14.8% (range 0–44.4) and 5.6% (range 0–23.3), respectively. shows biomarkers, clinical, echocardiographic, and exercise characteristics of the patients before and after CRT. Both systolic and diastolic blood pressure decreased significantly following nCRT, along with an increased heart rate. Weight dropped statistically significantly by a sample of median 0.6 kg [IQR (0–3)].
Table 1. Baseline characteristics before CRT.
Table 2. Patient characteristics, echocardiographic, and CPX changes from before CRT to after CRT.
Myocardial biomarkers and biochemistry
The levels of TNT and NT-ProBNP were within the normal range at baseline, and no significant changes in these biomarkers were noted after CRT. Subgroup analyses of the levels of TNT and NT-ProBNP among patients with clinically relevant impairment of LVEF and GLS did not differ from those of the patients with unchanged values of LVEF and GLS. No changes in cholesterols and triglycerides were observed from before CRT to after CRT.
Myocardial function at rest
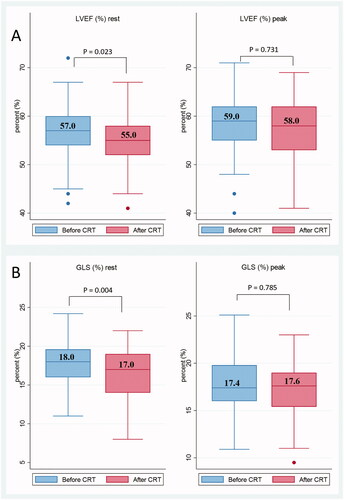
LV function was borderline abnormal before CRT with normal references of GLS > 18% and LVEF > 50%. Statistically significant decreases of LVEF and GLS were recorded at rest: 56.4 vs. 55.1% and 17.6 vs. 16.4%, respectively (p = 0.020 and p = 0.004). Absolute LVEF and GLS decrease of at least 5 and 2.5%, respectively, were noted in 21% (n = 10) after nCRT as shown in (A) GLS and (B) LVEF. LV diastolic dysfunction was observed in six patients (13%) before CRT, and in ten patients (21%) after CRT, this difference did not reach statistical significance (p = 0.41). RV systolic function evaluated by TAPSE and RV GLS remained unchanged and within normal limits.
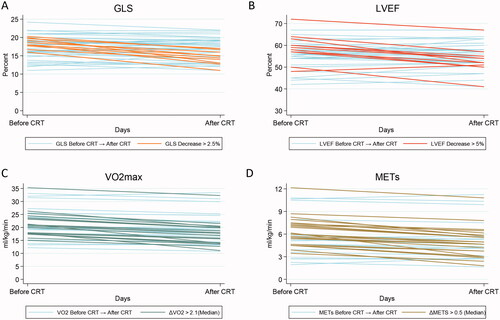
Figure 1. Changes in left ventricular (LV) function and physical performance following chemoradiotherapy (CRT). Two-way graphs displaying 47 individual patient plots of (A) Global longitudinal strain (GLS); (B) LV ejection fraction (LVEF); (C) Maximal oxygen consumption (VO2max); (D) metabolic equivalent of task (METs). The higlighted lines in each plot represents values of clinically relevant changes. Absolut decreases in LVEF > 5% and GLS > 2.5% were seen in 21% of the patients. A decrease > 2.1 ml/kg/min was seen in 47% of patients, and 43% of patients had a decrease in METs > 0.5 ml/kg/min.
Myocardial function during exercise
LV systolic reserve capacity evaluated by the differences in values of LVEF and GLS from rest to peak exercise is demonstrated in . Absolute increases in LVEF and GLS of 7–8 and 5–6%, respectively, from rest to peak exercise (ΔLVEF and ΔGLS) were expected [Citation9]. An inadequate rise in levels of GLS or LVEF was observed during exercise, hence demonstrating a reduced LV systolic reserve capacity both before and after CRT with absolute increases of only (a) 2–3% LVEF; (b) 0–1% GLS, as shown in in box plots with median values.
Physical performance in relation to myocardial dysfunction
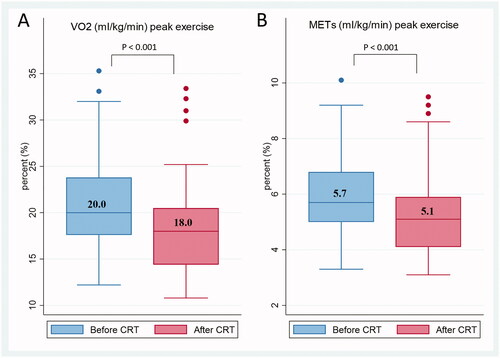
Maximal work capacity illustrated by VO2max and METs decreased significantly after CRT, by 21.2 vs. 18.8 and 5.9 vs. 5.4, respectively (p < 0.001 and p < 0.001). (A) VO2max; (B) METs show the decreases in box plots with median values. We found a weak correlation between change in GLS at peak and decrease in VO2max with r = 0.369 (p = 0.018). A significant correlation was noted between the decrease in VO2max and the decrease in hemoglobin with r = 0.333 (p = 0.033). No correlations were found between the decrease in weight and creatinine, or the decrease in VO2max and the decreases in weight, systolic and diastolic blood pressure, changes in GLS at rest, LVEF at rest, or LVEF at peak. shows the decreases in (C) VO2max; (D) METs. A total of 47% of patients had VO2max decreases above the median value of 2.1 ml/kg/min, and 43% of patients had METs decreases above the median value of 0.5 ml/kg/min.
Discussion
The main findings were as follows: (1) LV function decreased significantly at rest evaluated by GLS and LVEF. In fact, a clinically relevant decline in GLS (2.5%) and LVEF (5%) was seen in one-fifth of the patients; (2) LV systolic reserve capacity evaluated at peak exercise was significantly impaired before CRT and remained impaired after CRT and (3) physical performance decreased as shown by a significant reduction in maximal oxygen consumption after CRT, this reduction correlated with the GLS decrease at peak.
Myocardial dysfunction at rest
One-fifth of the patients had significantly impaired systolic function assessed from their cardiac response to CRT, which was evaluated immediately after the final treatment with a median F-U of one day. However, although reduced, LVEF stayed within the normal range (>50%). Inversely, GLS fell from a borderline abnormal level to a level that may be considered mildly reduced. Though the impairment of LV systolic function seems significant, LVEF did not drop to a level at which guidelines recommend initiation of heart failure treatment [Citation23]. Whether the decrease in LV systolic function observed will persist or increase the future risk of cardiovascular events needs to be established in future studies. However, the demonstrated decrease in LV systolic function was not accompanied by an increase in troponins, such as TNT. This suggests that myocyte necrosis was not involved. It may also indicate that the LV systolic changes might be temporary. In addition, the level of NT-ProBNP did not rise in response to the cardiac CRT exposure. This is consistent with the clinical observations that no patients had clinical signs of heart failure or experienced aggravated functional dyspnea during their CRT treatment course. Both systolic and diastolic blood pressure decreased statistically significantly. Under normal cardiac conditions, these decreases would result in a consequential LVEF increase, possibly indicating that the LVEF decrease has a more significant impact than initially assumed.
Myocardial function during exercise
To detect any additional subclinical LV systolic dysfunction that was not demonstrated by resting echo, the patients underwent stress exercise echo to define the LV systolic reserve capacity, expecting that LVEF and GLS would increase from rest to peak exercise [Citation8]. Evaluation of LV systolic reserve capacity has previously proved a valuable and reliable method for detecting various pathologic cardiac conditions [Citation24]. In the present study, both before and after CRT, LV systolic response to exercise was impaired with a diminished increase in LVEF and GLS. This reduced systolic reserve capacity may possibly be related primarily to their baseline cardiovascular risk profile as CRT treatment did not aggravate their LV response to exercise. To our knowledge, no previous study has evaluated the LV systolic reserve capacity in EC patients, and the demonstration of a reduced myocardial capacity may possibly have cardiac prognostic implications, as suggested by Paraskevaidis [Citation25].
Decrease in physical performance
Cardiopulmonary exercise capacity directly influences the affected patients' daily well-being and activity levels independently of cardiac disease. Among patients with heart failure of different etiology, the maximal cardiopulmonary exercise capacity is associated with morbidity and mortality [Citation11]. In the present study, the maximal work capacity expressed by VO2max, METs, and percent predicted VO2max decreased significantly. This finding aligns with the results of a Swedish study in which exercise capacity decreased from 150 W before CRT to 125 W after CRT [Citation26]. The percent predicted VO2max decreased from 87.5% before CRT to 76.8% after CRT, a fall in physical performance of 10.7% (absolute value) from before to after CRT. This measure provides age-sex and weight-adjusted values that can be used as an additional tool for detecting subtle cardiopulmonary limitations in EC cancer patients. The decrease in physical performance may adversely impact the subsequent post-operative risk of severe toxicity, prolong hospital stay, and further delay rehabilitation after surgery in the EC patients.
CRT and heart disease
The radiation dose levels demonstrated in the current study show that the tumors received the intended dose and that the surrounding critical organs, including the heart and the lungs, were kept within the recommended constraints. Previous studies have shown that such dose levels are related to possible cardiotoxic side effects, including clinical heart failure, arrhythmia, and pericardial effusion [Citation2,Citation27,Citation28]. However, the true clinically relevant lower radiation dose limit to the whole heart and heart substructures remains to be established in esophageal cancer patients. There is indirect proof of radiation-induced heart disease from a prospective phase-2 study in EC patients reporting that proton-based therapy was associated with a significant reduction in radiation dose to the heart compared to conventional photon CRT. Hence, a subsequent decrease in acutely diagnosed heart disease and post-operative lung complications was observed [Citation29]. Confirming results are awaited from two randomized phases 3 studies currently comparing proton therapy to standard photon radiotherapy in EC patients (NRG–GI006, ClinicalTrials.gov NCT03801876; PROTEC-trial, ClinicalTrials.gov NCT05055648) [Citation30,Citation31]. Paclitaxel is known to have potentially cardiotoxic side effects after long-term use (months to years). However, in the current regimen, the paclitaxel dose was low, 50 mg/m2; and the total dose was limited to five series, why severe cardiotoxic effects are less likely. Carboplatin is not known to have severe cardiotoxic side effects.
Limitations
This was a single-center study in which 47 of the 59 recruited patients were included. This drop-out represents the reality of early progression during diagnostic work-up. The patients studied here are fragile and undergo harsh treatment, and 20% were unable to complete the study program, which included cardiology assessments. Another limitation of this study is the lack of a control group, which was not feasible in EC patients. However, physical performance values were evaluated by reference to the study by Larsen et al., where a population of healthy individuals went through the same investigations as our patients [Citation9]. The present study only represents patients of Caucasian origin. Therefore, the results cannot be extrapolated directly to EC patients with other clinical characteristics, including different ethnicity [Citation32].
Perspectives
The focus within radiation-induced cardiac toxicity has been on late side effects occurring months to years after treatment in long-term survivors among patients with lymphoma and breast cancer [Citation33]. In contrast, this study on EC patients showed early effects of CRT on the cardiovascular system that may impact the patient’s capability to endure comprehensive curative surgery and subsequent rehabilitation or exacerbate cardiac morbidity. Clinicians need to be acquainted with these possible early cardiac side effects to follow and treat subsequent heart disease and, in the future to select patients for optimal and heart-sparing treatment to reduce cardiovascular morbidity and mortality. Further studies, including longer follow-up and serial cardiac examinations, are needed to determine if early cardiac changes subsequently turn into heart disease and to detect and institute appropriate treatment of such cardiac side effects.
Conclusion
LV systolic function and physical performance decreased in EC patients in the immediate post-treatment phase. The changes remained subclinical even though one-fifth of the patients experienced a significant reduction in LV systolic function. The cardiac reserve capacity was impaired; this reduction was related to the demonstrated decline in the maximal physical performance following CRT. This study highlights that EC treatment is associated with early cardiac side effects, which may have clinical and future prognostic implications.
Supplemental Material
Download MS Word (29.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets generated and analyzed during the current study are not publicly available due to the protection of personality, confidentiality, and privacy interests. Still, they are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Witt JS, Jagodinsky JC, Liu Y, et al. Cardiac toxicity in operable esophageal cancer patients treated with or without chemoradiation. Am J Clin Oncol. 2019;42(8):662–667.
- Yusuf SW, Venkatesulu BP, Mahadevan LS, et al. Radiation-induced cardiovascular disease: a clinical perspective. Front Cardiovasc Med. 2017;4:66.
- Lund M, Alexandersson von Döbeln G, Nilsson M, et al. Effects on heart function of neoadjuvant chemotherapy and chemoradiotherapy in patients with cancer in the esophagus or gastroesophageal junction – a prospective cohort pilot study within a randomized clinical trial. Radiat Oncol. 2015;10:16.
- Søndergaard MMA, Nordsmark M, Nielsen KM, et al. Cardiovascular burden and adverse events in patients with esophageal cancer treated with chemoradiation for curative intent. JACC CardioOncol. 2021;3:711–721.
- Tops LF, Delgado V, Marsan NA, et al. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. 2017;19(3):307–313.
- Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Pt A):2751–2768.
- Patel J, Rikhi R, Hussain M, et al. Global longitudinal strain is a better metric than left ventricular ejection fraction: lessons learned from cancer therapeutic-related cardiac dysfunction. Curr Opin Cardiol. 2020;35(2):170–177.
- Larsen AH, Clemmensen TS, Wiggers H, et al. Left ventricular myocardial contractile reserve during exercise stress in healthy adults: a two-dimensional speckle-tracking echocardiographic study. J Am Soc Echocardiogr. 2018;31(10):1116–1126.e1.
- Clemmensen TS, Løgstrup BB, Eiskjaer H, et al. Coronary flow reserve predicts longitudinal myocardial deformation capacity in heart-transplanted patients. Echocardiography. 2016;33(4):562–571.
- Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(2):101–138.
- Chiappa A, Andreoni B, Dionigi R, et al. A rationale multidisciplinary approach for treatment of esophageal and gastroesophageal junction cancer: accurate review of management and perspectives. Crit Rev Oncol Hematol. 2018;132:161–168.
- Bredy C, Ministeri M, Kempny A, et al. New York heart association (NYHA) classification in adults with congenital heart disease: relation to objective measures of exercise and outcome. Eur Heart J Qual Care Clin Outcomes. 2018;4(1):51–58.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
- Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196–1207.
- Okuhara Y, Asakura M, Orihara Y, et al. Reduction in left ventricular ejection fraction is associated with subsequent cardiac events in outpatients with chronic heart failure. Sci Rep. 2019;9(1):17271.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360.
- Nyeng TB, Nordsmark M, Hoffmann L. Dosimetric evaluation of anatomical changes during treatment to identify criteria for adaptive radiotherapy in oesophageal cancer patients. Acta Oncol. 2015;54(9):1467–1473.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14.
- Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69(8):1043–1056.
- Balady GJ, Arena R, Sietsema K, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225.
- McDonagh TA, Metra M, Adamo M, et al. Corrigendum to: 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2021;42(48):4901.
- Badran HM, Faheem N, Ibrahim WA, et al. Systolic function reserve using two-dimensional strain imaging in hypertrophic cardiomyopathy: comparison with essential hypertension. J Am Soc Echocardiogr. 2013;26(12):1397–1406.
- Paraskevaidis IA, Ikonomidis I, Simitsis P, et al. Multidimensional contractile reserve predicts adverse outcome in patients with severe systolic heart failure: a 4-year follow-up study. Eur J Heart Fail. 2017;19(7):846–861.
- von Döbeln GA, Nilsson M, Adell G, et al. Pulmonary function and cardiac stress test after multimodality treatment of esophageal cancer. Pract Radiat Oncol. 2016;6(3):e53–e9.
- Hayashi Y, Iijima H, Isohashi F, et al. The heart's exposure to radiation increases the risk of cardiac toxicity after chemoradiotherapy for superficial esophageal cancer: a retrospective cohort study. BMC Cancer. 2019;19(1):195.
- Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol. 2015;114(1):85–90.
- Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38(14):1569–1579.
- Møller DS, Poulsen PR, Hagner A, et al. Strategies for motion robust proton therapy with pencil beam scanning for esophageal cancer. Int J Radiat Oncol Biol Phys. 2021;111(2):539–548.
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):488–497.
- Delman AM, Ammann AM, Turner KM, et al. A narrative review of socioeconomic disparities in the treatment of esophageal cancer. J Thorac Dis. 2021;13(6):3801–3808.
- Nordsmark M, Offersen BV. The risk of radiation-associated heart disease comes from many factors; the chain is as strong as the weakest link. Radiother Oncol. 2020;152:101–102.