Abstract
Introduction
Long-term data on disease trajectory of EGFR-mutated early-stage non-small cell lung cancer (NSCLC) is still limited. This is relevant in the context of the recently approved introduction of adjuvant EGFR-targeting therapy, specifically osimertinib in resected stage II–III EGFR-mutated NSCLC.
Methods
Long-term data on patients with resected adenocarcinoma of the lung and known EGFR-status were analysed with focus on site of relapse and detailed cause of death. Patients resected in the period 2006 to 2018 were included.
Results
Of 503 patients (286 (57%) females, median age 67.3 years), 62 (12%) harboured an EGFR-mutation, 286 (57%) were in stage I. After a median follow-up of 8.0 years, 241 (48%) patients relapsed. Recurrence occurred in 30% and 53% of EGFR-positive stage IA and IB patients, respectively. Median overall survival was longer in EGFR-mutated versus non-mutated patients (128 versus 88 months). The recurrence rate, time to recurrence and rate of brain metastases was not different between EGFR-mutated and non-mutated groups. Median time from recurrence to death was longer in EGFR-mutated patients (31 months) compared with non-mutated patients (15 months). More patients without EGFR-mutation succumbed to non-cancer related death (18%) compared to patients with EGFR-mutations (8%).
Conclusions
The recurrence pattern in EGFR-mutated and non-mutated NSCLC-patients is similar and the rate is high in early stages. Time from recurrence to death and overall survival is longer in the EGFR-mutated group, due to lower risk of non-lung cancer deaths, and efficient treatment upon relapse.
Introduction
Lung cancer is the biggest cancer killer worldwide, and even for resected early-stage disease the prognosis is dismal with a reported five-year overall survival of around 50% [Citation1,Citation2]. Adjuvant chemotherapy after surgery increases five-year overall survival with around 5% in stage II and III [Citation3]. Around 10% of all non-squamous cell non-small cell cancers (NSCLC) harbour an activating mutation in the EGFR-gene in unselected western populations [Citation4,Citation5]. Recently, osimertinib was approved as adjuvant therapy in resected EGFR-mutated NSCLC, based on results from the ADAURA trial [Citation6]. In this trial, three-year treatment of osimertinib was shown to reduce risk of disease recurrence at two years with 80% in patients with resected NSCLC stage IB–IIIA harbouring ex19del or L858R EGFR mutation.
The recurrence rate and pattern of relapse in resected EGFR-mutated lung cancer compared with non-mutated cases is not well studied, especially not in a non-Asian population [Citation7–9]. Some data indicate a difference in response to osimertinib in Asians compared with non-Asians in advanced stages [Citation10]. It is known that advanced EGFR-mutated NSCLC has a high risk of brain metastases [Citation11], but the rate of such relapse in early stage is largely unknown. Osimertinib is shown to have a superior penetration into brain compared to earlier EGFR-inhibitors and thus is more effective in preventing brain metastases [Citation12]. It is thus hoped that adjuvant osimertinib may be effective in preventing brain metastases and increase survival in EGFR-mutated early-stage lung cancer.
Furthermore, since never-smokers are more prevalent in EGFR-mutated lung cancer than in non-mutated cases, smoking-related comorbidities may be less prevalent [Citation13]. Thus, overall survival may be different between the groups due to a lower rate of non-cancer related deaths in EGFR-mutated NSCLC, and not necessarily due to differences in biological features per se.
Here we have in detail analysed a cohort of resected lung adenocarcinoma with known EGFR-status and compared recurrence features and long-term outcome in EGFR-mutated cases with non-mutated cases. We have also studied histopathological features (differentiation grad and PD-L1-expression levels) and the efficacy of EGFR-TKI in treatment of recurrence of resected EGFR-mutated NSCLC.
Materials and methods
From 2006 to 2018, non-small cell lung cancer patients surgically resected at the Oslo University Hospital were included in a prospective study. Clinical and histopathological information was stored in a database and tissue was obtained and collected in a biobank. Clinical information during follow-up was entered in the database, and virtually no patient was lost to follow-up. Vital status was obtained from the National Population Register which is updated monthly. Oslo University Hospital is a public tertiary hospital, receiving patients from 8 secondary hospitals serving approximately 1 mill inhabitants. The project was approved by the institutional review board and regional ethics committee (ref: S-06402b), and written consent was obtained from all participants.
Norwegian lung cancer guidelines are updated regularly, and follow to a large extent European and other international guidelines. In this study, patients are staged (or for consistency re-staged if initially staged in other version) according to International Union Against Cancer (UICC) TNM staging version 7 (TNM7). Patients potentially eligible for curative treatment undergo PET-CT, but preoperative MRI of the brain is not standard of care. Adjuvant chemotherapy was routinely offered to patients in stage II-III aged 70 years or younger.
All cases were analysed with antibodies against TTF1 and p40 to discern between squamous carcinoma or adenocarcinoma, and PD-L1 expression level was scored using the 22C3 antibody according to standard methodology [Citation14].
EGFR-testing was done using either TheraScreen EGFR mutation kit (DxS, Manchester, UK), Cobas EGFR mutation PCR-test (Cobas v2; Roche Diagnostics, Pleasanton, CA) or using a next generation sequencing platform (IonTorrent Oncomine Comprehensive Assay, ThermoFisher, Waltham MA).
In this study, only patients with pure adenocarcinoma histology, known EGFR-status and in stage I-III were included. No patient has received osimertinib as (neo)adjuvant therapy, and none in recurrent situation except one T790M-negative in second line with no response.
Relapse free survival (RFS) and overall survival (OS) was calculated based on standard definitions, using date of surgery as time zero, and for OS all-cause deaths were events [Citation15]. Relapse-free cause-specific survival may represent the biological effect of an EGFR-mutation better than RFS. Relapse-free cause-specific survival is defined as time from surgery to either cancer-relapse or death caused by lung cancer, whichever comes first. This parameter, in contrast to RFS censors non-lung cancer related deaths thus avoiding bias due to differences in comorbidities between the groups. All patients were followed with 3-monthly recurrence status inquiries based on local hospital medical records and cut-off for analyses was May 1st 2021. Vital status was known for all patients at time of data cut-off.
Pearson’s chi-squared tests were used to assess differences between EGFR-mutated and EGFR non-mutated patients by differentiation grade and gender distribution, while a Mann–Whitney test was used to test the difference in mean PD-L1 expression. The Kaplan–Meier approach was used to estimate overall survival, relapse-free survival and relapse-free cause-specific survival. Competing risks [Citation16] were accounted for both when estimating the proportion dying from lung cancer, other causes and being alive, as well as, when estimating the proportion developing brain metastasis, other metastasis, local recurrence, death and being alive. Three multivariable Cox regression models were performed adjusted for case-mix, that is, EGFR-status, time period of resection, age group, gender, stage and pack years of smoking. An interaction between follow-up time and EGFR-status was used to account for non-proportionality. Follow-up times were defined as (1) time from surgery to date of death, (2) time from surgery to date of first development of metastasis, local recurrence or death, and (3) time from date of relapse to date of death. A p-value <0.05 was considered significant, and all statistical analyses were performed using Stata 17.0 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Patient characteristics
From a total cohort of 960 surgically resected NSCLC stage I-IIIb in the period 2006-2018, 503 patients with adenocarcinoma with known EGFR-status were included (). Of the patients, 56.9% were females, 0.6% were of non-Caucasian ethnicity, median age at time of surgery was 67.3 years, and 56.9%, 27.6% and 15.5% were in stage I, II and III, respectively. Median follow-up time was 8.0 years. Sixty-two patients (12.3%) were found to harbour EGFR-mutated tumours, 23 (37.1%) L858R and 23 (37.1%) exon 19 deletions, and 16 (25.8%) other variants (7 with exon 20 insertions, six with G719X-mutations in exon 18 including one with concomitant S768I in exon 20, and 3 with L861Q-mutations in exon 21). Of the 283 PD-L1-tested tumours, 28.3% were scored as positive (≥ 1%), mean PD-L1 level was 11.9%, and 11.0% of tumours had a PD-L1-expression level of 50% or more. The most frequent operation type was lobectomy in 368 (73.2%) of cases, followed by bilobectomy in 55 (10.9%), sublobar resection in 45 (8.9%) and pneumonectomy in 35 (7.0%) of patients.
Table 1. Baseline characteristics of total patient group and patients with EGFR-mutated tumours (EGFR mut) and non-EGFR-mutated tumours (EGFR non-mut), respectively.
When comparing EGFR-mutated and non-mutated patients we found significantly more never smokers and females in the mutated group (). Of tumours with defined differentiation grade (83.9% of all), more EGFR-mutated tumours were highly differentiated compared with non-mutated tumours (20.8 versus 11.4%) and fewer had low differentiation grade (17.0 versus 29.8%), but this did not reach statistical significance (p = 0.099). EGFR-mutated tumours had a lower mean PD-L1 expression level compared with non-mutated tumours (4.6 versus 13.3%) (p = 0.007). Median level of expression was 0% in both mutated, non-mutated and the total cohort.
Out of 128 non-mutated cases eligible for adjuvant chemotherapy (≤70 years, stage II-III) 94 of 128 (73.4%) received (neo-)adjuvant chemotherapy compared with 11 of 14 (78.6%) eligible mutated cases. None received (neo-)adjuvant EGFR-TKI. A total of 23 (4.6% of all) patients received (neo-) adjuvant radiotherapy, 3 of these harboured EGFR-mutated tumours (4.8% of the EGFR-mutated cohort).
Outcome
There was no difference in relapse rate between EGFR-mutated and non-mutated patients, and the rate of brain metastasis was also similar (). Patients with EGFR-mutations had a significantly longer OS than patients without EGFR-mutation (128 versus 87.5 months, p = 0.0118) ( left). When excluding patients in stage Ia, median OS was 92.3 versus 72.5 months (p = 0.090). Median survival among patients in stage II and III was 70.4 and 48.9 months for patients with and without mutations, respectively (p = 0.115) (data not shown). Patients with EGFR-mutations had a numerically longer RFS, but this did not reach statistical significance ( middle). Relapse-free cause-specific survival was similar in EGFR-mutated and non-mutated patients (median TTR 87.7 versus 87.3 months respectively), but there was a numerical delay in time to recurrence in EGFR-mutation positives from 1 to 4 year after surgery ( right).
Figure 1. Overall survival (A, left), Relapse-free survival (A, middle), Relapse-free cause-specific survival (A, right) and Cause of death (B) among EGFR-mutated and -nonmutated surgically resected lung adenocarcinoma. Time zero is date of surgery.
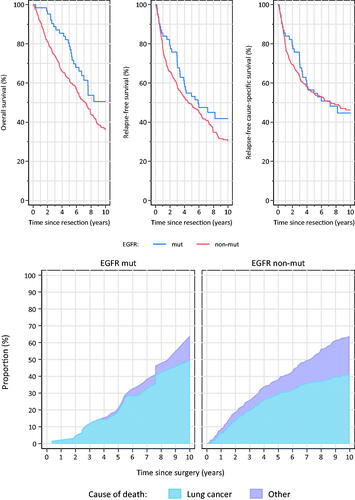
Patients without mutation had a higher risk of dying of non-cancer related deaths (). There was a numerically longer median relapse-free cause-specific survival in patients with L858R compared with exon 19-mutations and other mutations (99.8 vs 87.7 vs 48.8 months respectively), but this did not reach statistical significance (data not shown).
In multivariate analyses, OS-difference between EGFR-mutated and non-mutated patients was significant in the first 2-year period after surgery, but not later (). Patients resected in the first study period (2006–2011), and being 75 years or older had a significantly worse OS (p = 0.049 and 0.005 respectively). Patients in stage II and III had a 66% and 129% increased risk of death compared with patients in stage I. Relapse-free survival was statistically significant associated with stage, and overall survival (but not relapse-free survival or survival after relapse) was significantly associated with number of pack-years (p = 0.022 per 1 year increment). Risk of death after relapse was significantly lower in EGFR-mutated patients the first 2 years after relapse, whereas thereafter the EGFR-mutated patients had a 2-fold higher risk of death. Furthermore, patients resected in the former time period, and males, had a significantly shorter time from relapse to death, whereas stage and age did not impact on the time from relapse to death in these multivariate analyses.
Table 2. Multivariate analyses of overall survival, relapse free survival and survival after relapse.
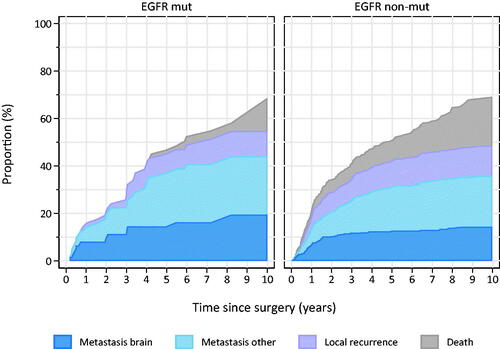
Patients with EGFR-mutation had lower propensity of local recurrence (ipsilateral hemithorax/mediastinum), and a lower risk of death compared with the non-mutated group (). There was no significant difference in pattern of brain metastases development between the groups. Median time to detection of brain metastases was numerically longer in EGFR-mutated patients (36.9 vs 21.1 months for EGFR-mutated and non-mutated, respectively), but this difference was non-significant. At 18 months, 5% of EGFR-mutated and 7% of non-mutated patients had developed brain metastases. For both mutated and non-mutated patients, the risk of developing brain metastases in the study period increased with stage, and close to 1/3 of patients in stage III developed brain metastases. Overall, around 2% of patients had brain metastases diagnosed within 6 months after surgery, in stage III this number increased to 6%.
Figure 2. Relapse pattern among EGFR-mutated and -nonmutated surgically resected lung adenocarcinoma.
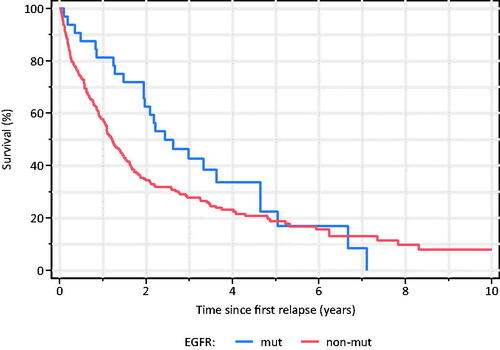
Median time from first relapse to death was significantly longer in EGFR-mutated patients (30.8 vs 14.9 months, p = 0.0080) (). This difference was also seen in the subgroup of patients that developed brain metastases (not shown).
Figure 3. Time from first relapse to all-cause death among EGFR-mutated and -nonmutated surgically resected lung adenocarcinoma.
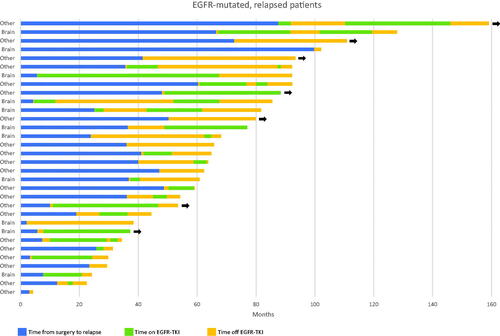
We also collected detailed information on treatment history for the 32 EGFR-mutated patients that relapsed (, Supplementary Table 1). Twenty-three of these received EGFR-TKI. None of the patients received osimertinib, except one T790M-negative who was put on osimertinib with no response, after relapse on gefitinib. Median time on EGFR-TKI was 13.1 months. Among the 11 with brain metastases, nine were treated with EGFR-TKI with a median time on TKI treatment of 22.9 months. Most of these had also received brain irradiation (whole brain or stereotactic radiotherapy) and/or surgical metastasectomy.
Discussion
In this study of 503 resected early-stage NSCLC patients of mainly north-European ethnicity, the EGFR-mutation rate was as found in advanced stage cohorts. The relapse rate was similar between EGFR-mutated and non-mutated patients, but the overall survival was longer among EGFR-mutated patients due to lower propensity of non-cancer deaths and a longer post-relapse survival.
The EGFR-positivity rate (12.3%) was in line with what has been published for early-stage NSCLC in western populations [Citation17,Citation18]. There was no difference in age or stage distribution between mutated and non-mutated groups. The frequency of uncommon mutations in our data set is somewhat higher than expected based on studies on advanced cancer, as 25.8% of EGFR-positive tumours were of the non-L858R/non-ex19del variants. In a study by Kerr et al, 16.9% of EGFR-mutated early-stage adenocarcinoma cases harboured non-L858R/non-ex19del mutations [Citation17]. Another study found exon20-insertion mutations in 12% of EGFR-mutant cases, but stage distribution was not reported [Citation19].
The expression of PD-L1 was substantially lower in mutated versus non-mutated tumours, in line with what has been found in advanced NSCLC. PD-L1-levels are generally lower in early stage than in advanced lung cancer [Citation20], but the relation to EGFR-mutations in early stage has to our knowledge not been clearly defined. This finding may be of relevance when considering adjuvant immunotherapy, which may be of low benefit in EGFR-mutated tumours given the correlation of effect of atezolizumab to PD-L1 level in the Impower010 trial [Citation21].
Patients with EGFR-mutated tumours had a similar rate of relapse including risk of brain metastases as compared with non-EGFR-mutated cases, in contrast to some other studies [Citation22]. Patients with EGFR-mutated tumours had a lower risk of non-cancer related deaths, and survived longer after relapse than patients without EGFR-mutations likely due to more effective treatment options. The fact that patients with EGFR-mutated tumours were less prone to smoke has undoubtedly contributed to the lower risk of non-lung cancer, but other smoking-related, deaths. Recently, some studies have also shown similar DFS in EGFR-mutated and non-mutated cases and longer OS in EGFR-mutated cases, although cause-specific deaths were not reported [Citation7,Citation8]. A recent meta-analysis that indicate longer RFS and OS in EGFR-mutated patients may be hampered by non-complete adjustment of these facts [Citation23]. Of note, the EGFR-mutated patients in our study had a 2-fold lower risk of death the first two years after relapse, whereas this risk was 2-fold higher in patients surviving more than 2 years after relapse. This is likely due to the death-postponing effect of EGFR-TKIs.
Interestingly, we found a non-statistically significant longer time to recurrence in L858R-mutated cases compared to patients harbouring deletions in exon 19, which may indicate that the latter genotype leads to a somewhat more aggressive phenotype. This may underscore the different effect on EGFR-TKIs on these mutation subtypes in advanced disease, as deletions in exon 19 is associated with a longer duration of response to both first- and third-generation TKIs [Citation24,Citation25].
RFS in EGFR-mutated disease was in our dataset 79% at 2 years and 53% at 5 years. Two-year DFS in the placebo-arm of the overall population in the ADAURA trial was 52%, but in this trial patients in stage Ia were excluded [Citation6]. In our study, 30% of patients in stage Ia had recurred during the follow-up time.
In the ADAURA trial, no overall survival data are presented, and the presented follow-up time is relatively short. In our study, EGFR-mutated patients had a median OS of over 10 years, and over 7 years if excluding patients in stage Ia. None of the preceding trials testing EGFR-TKI as adjuvant treatment in unselected patients yielded OS gains [Citation26,Citation27], and neither have trials randomising between first-generation EGFR-TKI or chemotherapy in EGFR-mutated patients [Citation28–31]. The IMPACT-trial reported a five-year OS of 78% among EGFR-mutated patients in stage II–III, which is close to our finding of 70.4% in patients with similar characteristics.
In our dataset, CNS-relapse was seen in 17.7% of EGFR-mutated cases and in 16.1% of non-mutated patients over the whole study period. In the AUDARA-trial 10% of the control group developed brain metastases after a median follow-up time of 14.9 months. A limitation of both our study and the ADAURA trial is the lack of routine brain MRI in staging and follow-up of the patients.
The AUDARA trial included only the common EGFR-mutations; deletions in exon 19 and L858R-mutations in exon 21. Given the relative high frequency of other mutations, both in our and other datasets [Citation19], it will be of interest to study the effect of osimertinib or other inhibitors in this ‘uncommon’ group. Osimertinib may have effect on some uncommon mutations [Citation32], and other compounds as mobocertinib and amivantamab have shown effectivity in advanced NSCLC harbouring insertions in exon 20 [Citation33,Citation34].
As a high fraction of EGFR-mutated tumours do not recur, a finding corroborated in other studies [Citation6–9], it is paramount to identify patients that will not benefit from adjuvant therapy. Future work thus has to include studies on possible predictors of relapse as co-mutations, miRNA-profiles and proteomic features [Citation35–37], as well as the usefulness of circulating tumour DNA in follow-up [Citation38]. Ongoing studies on the feasibility of EGFR-TKI as neoadjuvant therapy like the phase III NeoADAURA (NCT04351555) will also increase our knowledge of the optimal handling of early-stage NSCLC-patients with EGFR-mutated tumours.
Conclusions
In conclusion, we found that risk of recurrence in early-stage NSCLC seems unrelated to EGFR-mutational status, but overall survival is longer in EGFR-mutated disease due to a combined effect of lower risk of non-cancer related deaths, and better treatment upon relapse.
Author contributions
Inger Johanne Z. Eide: Conceptualisation, Methodology, Investigation, Writing - original draft preparation. Yngvar Nilssen: Methodology, Formal analysis, Visualisation, Writing – Review and editing. Marius Lund-Iversen: Investigation, Resources, Data curation, Writing – Review and editing. Odd Terje Brustugun: Conceptualisation, Supervision, Project administration, Funding acquisition, Writing – Review and editing.
Supplemental Material
Download MS Word (13 KB)Acknowledgements
The expert logistical and technical help from Ingjerd Solvoll is highly appreciated.
Disclosure statement
OTB: Consulting or advisory role: AstraZeneca, Roche, BoehringerIngelheim; research funding: AstraZeneca, Pfizer. The remaining authors have declared no conflicts of interest.
Additional information
Funding
References
- Pignon JP, Tribodet H, Scagliotti GV, LACE Collaborative Group, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26(21):3552–3559.
- Goldstraw P, Chansky K, Crowley J, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51.
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the lung adjuvant cisplatin evaluation. J Thorac Oncol. 2010;5(2):220–228.
- Skov BG, Høgdall E, Clementsen P, et al. The prevalence of EGFR mutations in non-small cell lung cancer in an unselected caucasian population. APMIS. 2015;123(2):108–115.
- Helland Å, Skaug HM, Kleinberg L, et al. EGFR gene alterations in a norwegian cohort of lung cancer patients selected for surgery. J Thorac Oncol. 2011;6(5):947–950.
- Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-Mutated Non-Small-Cell lung cancer. N Engl J Med. 2020;383(18):1711–1723.
- Saw SPL, Zhou S, Chen J, et al. Association of clinicopathologic and molecular tumor features with recurrence in resected Early-Stage epidermal growth factor Receptor-Positive Non-Small cell lung cancer. JAMA Netw Open. 2021;4(11):e2131892.
- Galvez C, Jacob S, Finkelman BS, et al. The role of EGFR mutations in predicting recurrence in early and locally advanced lung adenocarcinoma following definitive therapy. Oncotarget. 2020;11(21):1953–1960.
- Ni J, Guo T, Li Y, et al. Patterns and risks of postoperative recurrence in completely resected EGFR-mutant non-small cell lung cancer: prognostic significance of routine immunohistochemical markers. Transl Lung Cancer Res. 2019;8(6):967–978.
- Ito K, Morise M, Wakuda K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6(3):100115.
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111.
- Colclough N, Chen K, Johnström P, et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27(1):189–201.
- Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126(2):347–351.
- Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24(6):392–397.
- Punt CJ, Buyse M, Köhne CH, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99(13):998–1003.
- Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statist Med. 1999;18(6):695–706.
- Kerr KM, Dafni U, Schulze K, ETOP Lungscape Consortium, et al. Prevalence and clinical association of gene mutations through multiplex mutation testing in patients with NSCLC: results from the ETOP lungscape project. Ann Oncol. 2018;29(1):200–208.
- Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3(2):111–116.
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR exon 20 insertions and Co-Occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560–1568.
- Yu H, Brustugun OT, Ekman S, et al. Programmed cell death ligand 1 expression in resected non-small cell lung cancer. Clin Lung Cancer. 2021;22(4):e555–e562.
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357.
- Ito M, Miyata Y, Kushitani K, et al. Increased risk of recurrence in resected EGFR-positive pN0M0 invasive lung adenocarcinoma. Thorac Cancer. 2018;9(12):1594–1602.
- Zhang SM, Zhu QG, Ding XX, et al. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: a systematic review and Meta-analysis. CMAR. 2018;10:3393–3404.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-Mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33(17):1958–1965.
- Eide IJZ, Helland Å, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study). Lung Cancer. 2020;143:27–35.
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320–3326.
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA Non-Small-Cell lung cancer (RADIANT): a randomized, Double-Blind, phase III trial. J Clin Oncol. 2015;33(34):4007–4014.
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39(7):713–722.
- Tada H, Mitsudomi T, Yamanaka T, West Japan Oncology Group, et al. Randomized phase III study of gefitinib versus cisplatin plus vinorelbine for patients with resected stage II-IIIA Non-Small-Cell lung cancer With. J Clin Oncol. 2021;39(15_suppl):8501–8501.
- He J, Su C, Liang W, et al. Icotinib versus chemotherapy as adjuvant treatment for stage II-IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial. Lancet Respir Med. 2021;9(9):1021–1029.
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018;6(11):863–873.
- Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with Non-Small-Cell lung cancer harboring uncommon EGFR mutations: a multicenter, Open-Label, phase II trial (KCSG-LU15-09). J Clin Oncol. 2020;38(5):488–495.
- Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in Platinum-Pretreated patients with EGFR exon 20 Insertion-Positive metastatic Non-Small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021;7(12):e214761.
- Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 Insertion-Mutated Non-Small-Cell lung cancer progressing on platinum chemotherapy: Initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–3402.
- Liu SY, Bao H, Wang Q, et al. Genomic signatures define three subtypes of EGFR-mutant stage II-III non-small-cell lung cancer with distinct adjuvant therapy outcomes. Nat Commun. 2021;12(1):6450.
- Lehtiö J, Arslan T, Siavelis I, et al. Proteogenomics of non-small cell lung cancer reveals molecular subtypes associated with specific therapeutic targets and immune evasion mechanisms. Nat Cancer. 2021;2(11):1224–1242.
- Halvorsen AR, Ragle Aure M, Õjlert Å, et al. Identification of microRNAs involved in pathways which characterize the expression subtypes of NSCLC. Mol Oncol. 2019;13(12):2604–2615.
- Waldeck S, Mitschke J, Wiesemann S, et al. Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer. Mol Oncol. 2022;16(2):527–537.