Abstract
Background
Dosimetric and clinical comparison of two cohorts of Iridium-192 (Ir-192) and Cobalt-60 (Co-60) high-dose-rate brachytherapy (DR-BT) boost for localized prostate cancer.
Material and methods
Patients with localized prostate cancer receiving either Ir-192 or Co-60 high-dose-rate brachytherapy (HDR-BT) boost in combination with external beam radiotherapy (EBRT) in the period of 2002–2019 were evaluated for dosimetric differences, side effects, biochemical relapse-free survival (bRFS), metastasis-free survival (MFS), and overall survival (OS). EBRT, delivered in 46 Gy (DMean) in conventional fractionation, was followed by two fractions HDR-BT boost with 9 Gy (D90%) 2 and 4 weeks after EBRT. Genitourinary (GU)/gastrointestinal (GI) toxicity were evaluated utilizing the Common Toxicity Criteria for Adverse Events version 5.0 and biochemical failure was defined according to the Phoenix definition.
Results
A total of 338 patients with a median follow-up of 101.8 (IQR 65.7–143.0) months were evaluated. At 10 years the estimated bRFS, MFS, and OS in our patient sample were 81.1%/71.2% (p=.073), 87.0%/85.7% (p=.862), and 70.1%/69.7% (p=.998) for Ir-192/Co-60, respectively. Cumulative 5-year late grade ≥2 GU toxicity was 20% for Ir-192 and 18.3% for Co-60 (p=.771). Cumulative 5-year late grade ≥2 GI toxicity was 5.8% for Ir-192 and 4.6% for Co-60 (p=.610). Grade 3 late GU side effects were pronounced in the Ir-192 cohort with 8.1% versus 1.4% in the Co-60 cohort (p=.01), which was associated with significantly lower dose to the organs at risk in the Co-60 cohort. PTV D90% was 9.3 ± 0.8 Gy versus 9.0 ± 1.1 Gy (p=.027) for Ir-192 versus Co-60. PTV V100% and PTV V150% were not significantly different between both cohorts.
Conclusion
Co-60 brachytherapy sources are an effective alternative to Ir-192 in combined prostate HDR-BT boost + EBRT.
Introduction
High-dose-rate brachytherapy (HDR-BT) in combination with external beam radiotherapy (EBRT) is an established treatment option for localized prostate cancer. Due to the small size of the sources, Iridium-192 (Ir-192) is often preferentially utilized in interstitial HDR-BT. Since Cobalt-60 (Co-60) sources with identical dimensions to those of Ir-192 have been made available, its physical properties make the radionuclide Co-60 an interesting alternative in clinical brachytherapy [Citation1–3]. Co-60 has a longer half-life compared to Ir-192 with 63.3 months versus 2.4 months leading to a reduced number of source exchanges and therefore a significant cost reduction. As a tradeoff, Co-60 has a higher mean energy with 1.25 MeV compared to 0.37 MeV for Ir-192 resulting in the need for increased machine and room shielding. Moreover, Co-60 has a lower equivalent dose rate in air by a factor of 2.8 resulting in increased treatment times and the higher mean energy of Co-60 raises the concern about possible increased toxicity compared to patients treated with Ir-192. Therefore, in this current publication, the long-term oncological outcome, gastrointestinal (GI) and genitourinary (GU) toxicity, and dosimetric properties of two large real-life cohorts of prostate cancer patients treated with Ir-192 or Co-60 HDR-BT boost are compared.
Material and methods
Study design and participants
This retrospective single-center analysis is based on 338 consecutive male patients treated between 2002 and 2019 with combined 2-weekly HDR-BT boost after EBRT for localized prostate cancer. The characteristics and outcome for the whole cohort as well as the treatment regime have been described in an earlier publication in detail and shall be summarized in the following sections shortly [Citation4]. All patients had pathologically confirmed prostate cancer and were stratified into risk groups according to D’Amico et al. [Citation5]. Additive androgen deprivation therapy was recommended for patients with intermediate-risk (6 months) and high-risk disease (24–36 months) and prescribed at the discretion of the treating urologist. Ethical approval was waived by the local Ethics Committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. Informed consent was obtained from all individual participants included in the study.
Treatment
EBRT was delivered in 23 fractions with 2 Gy per fraction, resulting in a prescribed planning target volume (PTV) dose of 46 Gy. A clinical target volume (CTV) was generated consisting of the prostate and the seminal vesicles. The PTV was created by a 10 mm margin around the CTV in all but the dorsal direction, where a 7 mm margin was used. Pinnacle3 (Philips Radiation Oncology Systems, Fitchburg, WI, USA) was used for treatment planning. Approximately 2 weeks after completion of EBRT, two HDR-BT boost fractions were performed with a 14-d interval between the two applications. For each session, a new transperineal catheter implantation was performed with 3D TRUS-guided online planning in lithotomy position in general or spinal anesthesia. From 2002 to 2008, Ir-192 brachytherapy sources with the Nucletron PLATO system were used. Since 2008, Co-60 sources together with a Multi-Source and SagiNova HDR afterloader (Eckert & Ziegler BEBIG GmbH) in combination with the treatment planning systems HDRplus and SagiPlan (Eckert & Ziegler BEBIG GmbH) were utilized. The HDR-BT boost PTV was defined as the entire prostate without the seminal vesicles and additional margin. The prescription dose for the PTV was 9 Gy (D90%) per fraction. Selected properties of the two different radionuclides are summarized in Supplementary Table 1.
Outcome
Biochemical relapse-free survival (bRFS) was defined as the time between the end of radiotherapy and the date of biochemical failure. Biochemical failure was defined according to the Phoenix definition as nadir plus a ≥ 2 ng/ml increase in the prostate-specific antigen (PSA). Metastasis-free survival was defined as the time between the end of radiation therapy and the date of occurrence of distant metastasis, diagnosed by imaging. The time between the end of treatment and the date of death from any cause was defined as overall survival. Patients alive or lost to follow-up at the time of analysis were censored at the date of the last contact. Follow-up was defined as the time between the date of the end of radiation therapy and the date of last news. Assessment of physician-recorded toxicity during radiation therapy was performed at baseline, at the end of the treatment, 6 weeks after treatment, and in 6 months intervals thereafter. After 2 years, follow-up was changed to longer periods with annual examinations. GI and GU toxicity were scored using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Acute toxicity was defined as occurring between the start of radiation therapy and until three months after the end of radiation therapy. All subsequent follow-ups were included in the late toxicity evaluation.
Dosimetric evaluation
PTV coverage of the Ir-192 and Co-60 brachytherapy cohorts were evaluated for D90% (Gy), D100% (Gy), V100% (%), V150% (%), V200% (%). The conformal index (COIN) was defined according to Baltas et al. and calculated as the product of the fraction of PTV that is enclosed by the reference dose and the fraction of the reference dose volume that is covered by PTV [Citation6]. The organ at risk (OAR) rectum was evaluated for D1% (Gy), D10% (Gy), Dmax (Gy), D2cm³ (Gy), and the OAR urethra for D1% (Gy), D10% (Gy), Dmax (Gy), D0.1 cm³ (Gy).
Statistical considerations
Estimated bRFS, MFS, and OS were determined by the Kaplan–Meier method with associated log-rank testing for significant differences between Ir-192 and Co-60. Fisher`s exact tests were utilized for the comparison of toxicity between the two radionuclides. The statistical significance of the dosimetric differences between the two brachytherapy cohorts was tested by Mann–Whitney-U tests. Differences were considered statistically significant in the case of a two-sided p value of <.05. Statistical analysis was conducted using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics
A total of 120 patients received an HDR-BT boost with an Ir-192 source and 218 patients with a Co-60 source. The median follow-up of the whole cohort, consisting of 338 patients, was 101.8 (interquartile range 65.7–143.0) months. The clinical characteristics of both HDR-BT cohorts are summarized in .
Table 1. Clinical characteristics.
Dosimetric analysis
PTV D90% was 9.3 ± 0.8 Gy versus 9.0 ± 1.1 Gy (p = .027) for Ir-192 versus Co-60. PTV V100% and PTV V150% were not significantly different between both cohorts. The dose to the OARs rectum and urethra were significantly lower in the Co-60 cohort: Mean rectum D2cm³ was 6.4 ± 0.9 Gy versus 5.2 ± 0.7 Gy (p < .001) for Ir-192 versus Co-60. Mean urethra D0.1 cm³ was 13.5 ± 3.5 Gy versus 10.6 ± 1.1 Gy (p < .001), respectively. Target coverage for the PTV and the dose to the organs at risk rectum and urethra are summarized in .
Table 2. Dosimetric Comparison between Ir-192 and Co-60.
Oncologic outcome and toxicity
Biochemical relapse occurred in 25 (20.8%) patients with Ir-192 HDR-BT and 47 (21.6%) patients with Co-60 HDR-BT during follow-up. A total of 16 (13.3%) patients in the Ir-192 and 21 (9.6%) patients in the Co-60 cohort developed distant metastases. A total of 72 (61%) patients in the Ir-192 and 49 (23%) patients in the Co-60 cohort died during follow-up. BRFS, MFS, and OS were not statistically significant between both treatment cohorts. The estimated bRFS, MFS, and OS at 5 years was 87.0%/83.1%, 92.9%/93.6%, and 90.6%/89.8% for Ir-192/Co-60, respectively. At 10 years, the estimated bRFS, MFS and OS in our patient sample was 81.1%/71.2% (p = .073), 87.0%/85.7% (p = .862), and 70.1%/69.7% (p = .998) for Ir-192/Co-60, respectively. shows the estimated bRFS for the Ir-192 and Co-60 cohorts. Parameters for Cox proportional-hazards model analysis were HDR-BT source, stage (≤T2b; ≥T2c), Gleason score (≤7a; ≥7b), initial PSA (continuous variable), age at radiation therapy start (continuous variable), and androgen deprivation therapy. Gleason score was prognostic for bRFS, MFS, and OS. Initial PSA was a prognostic factor for bRFS. Age was prognostic for OS. HDR-BT source was not prognostic for bRFS, MFS, and OS. The results of the Cox proportional-hazards model analysis are summarized in .
Figure 1. Biochemical relapse-free survival. Shown is the estimated biochemical relapse-free survival for the Ir-192 group and the Co-60 group. Biochemical relapse-free survival was not significantly different between both groups (p=.073, log-rank test).
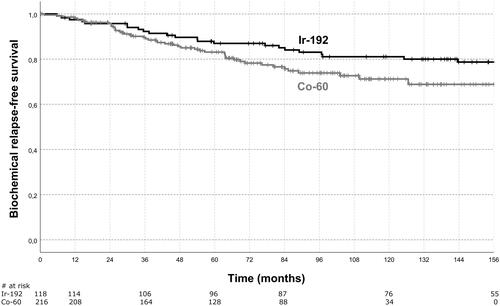
Table 3. Cox proportional-hazards model analysis.
No significant differences in acute toxicity between Ir-192 and Co-60 were observed. Cumulative acute grade ≥ 2 GU toxicity was 12.7% for Ir-192 and 11.6% for Co-60 (p = .860). Cumulative acute grade ≥ 2 GI toxicity was 2.5% for Ir-192 and 2.8% for Co-60 (p = .860). Cumulative 5-year late grade ≥ 2 GI toxicity was 5.8% for Ir-192 and 4.6% for Co-60 (p = .610). Cumulative 5-year late grade ≥ 2 GU toxicity was 20% for Ir-192 and 18.3% for Co-60 (p = .771). Cumulative 5-year late grade 3 GI toxicity was 0.8% for Ir-192 and 0% for Co-60 (p = .355) with one case of grade 3 rectal hemorrhage. Rectum D1%, D10%, Dmax, D2cm³ were not associated with increased severe GI toxicity (grade 3).
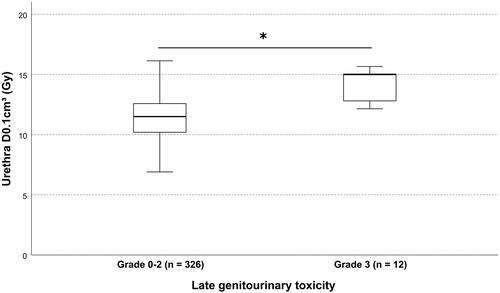
Late severe GU toxicity (grade 3) was increased in the Ir-192 cohort: Cumulative 5-year late grade 3 GU toxicity was 8.1% (n = 9) for Ir-192 and 1.4% (n = 3) for Co-60 (p = .01). In total, 12 patients experienced severe GU toxicity in the form of grade 3 urinary tract obstruction with one patient developing additional grade 3 non-infective cystitis and urinary incontinence. Urethral dose D1% (p = .0027), D10% (p = .04) were significantly associated with GU toxicity. Urethral D0.1 cm³ was increased in patients with grade 3 late GU toxicity with 14.1 ± 1.4 Gy versus 11.8 ± 2.9 Gy (p = .002), .
Figure 2. Urethral dose and late genitourinary toxicity. Shown is the box plot of the urethral dose D0.1cm³ in relation to the cumulative 5-year late genitourinary toxicity. box = interquartile range; solid horizontal line = median; whiskers = 1.5 × interquartile range. * indicates statistical significance (p=.002).
Discussion
Dose escalation of EBRT by HDR-BT boost is commonly prescribed for intermediate and high-risk prostate cancers, recommended by the current GEC-ESTRO ACROP prostate brachytherapy guidelines and most often applied as Ir-192 brachytherapy [Citation7]. To the best of our knowledge, this study is the first one comparing the oncologic outcome and side effects of Ir-192 with Co-60 HDR-BT boost for localized prostate cancer. Ir-192 and Co-60 sources have been compared for uterine cancers in the literature and several planning studies evaluated the physical properties and dose distribution of both sources and reported equivalence [Citation1,Citation8–12]. Only one study by Tantivatana et al. reported clinical endpoints for a retrospective cohort of 480 cases of cervical cancer patients: Cervical cancer patients who were treated with Co-60 HDR-BT were comparable in survival and toxicity outcomes to those with Ir-192 HDR-BT [Citation13]. In the randomized trial by Hoskin et al. for localized prostate cancer, a 12 year - bRFS of 48% for EBRT + HDR-BT boost was recently reported [Citation14]. With an estimated 10-year bRFS of 81.1%/71.2% (p = .073) for Ir-192/Co-60 and a cumulative 5-year late grade 3 GU/GI toxicity of 3.6%/0.3% our outcome and toxicity data are comparable to the published literature [Citation4,Citation14–18]. Overall, for localized prostate cancer HDR-BT boost, our data show no significant differences in bRFS, MFS, and OS between both source types. The non-significant trend to decreased 10 year - bRFS in the Co-60 cohort with an absolute difference of 9.9% (p = .073) may be the result of differences in underlying disease and dosimetric characteristics. PTV D90% was 9.3 ± 0.8 Gy versus 9.0 ± 1.1 Gy (p = .027) and PTV V200% was 20.4 ± 5.8% versus 18.1 ± 4.8% (p < .001) for Ir-192/Co-60. Even though D`Amico risk class as well as ADT use was balanced between both cohorts, the Co-60 cohort showed higher PSA at diagnosis and worse Gleason-scores (see also ). Moreover, differences in treatment planning systems and manufacturers due to the long timeframe of our study may have introduced additional bias. Severe late GU side effects were pronounced in the Ir-192 cohort with 8.1% versus 1.4% in the Co-60 cohort (p = 0.01). A possible explanation is the decreased urethral dose in the Co-60 cohort, which may have resulted from increasing experience in HDR-BT appliance. Grade 3 GU toxicity was significantly associated with increased urethral dose. Moreover, Richter et al. demonstrated for the same PTV dose that the integral dose is lower for Co-60 sources within a radius of about 20 cm compared to Ir-192 [Citation1]. Beyond this distance, the ratio reverses gradually due to the higher photon energy of Co-60. This higher energy requires more room shielding for the application of the Co-60 source [Citation1]. Source replacements are required for Co-60 every 5–6 years compared to every 2–3 months with Ir-192 sources, leading to significant cost-saving opportunities. Together with clinical equivalent outcomes and low side effects, Co-60 sources are a suitable alternative to Ir-192 sources in the context of HDR-BT boost for localized prostate cancer. This study is limited by its retrospective design with inherent differences in baseline and treatment characteristics but contributes first clinical results to the body of evidence in favor of utilizing Co-60 sources in HDR-BT for localized prostate cancer. Prospective, confirmatory studies are required to further evaluate Co-60 sources for prostate brachytherapy.
Conclusion
No significant differences in bRFS, MFS, and OS between the Ir-192 and Co-60 brachytherapy cohorts could be detected. Severe GU side effects were pronounced for higher urethral doses. Co-60 brachytherapy sources are an effective alternative to Ir-192 sources in combined prostate HDR-BT boost + EBRT.
Supplemental Material
Download MS Word (19.5 KB)Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Richter J, Baier K, Flentje M. Comparison of 60cobalt and 192iridium sources in high dose rate afterloading brachytherapy. Strahlenther Onkol. 2008;184(4):187–192.
- Islam MA, Akramuzzaman MM, Zakaria GA. Dosimetric comparison between the microSelectron HDR (192)Ir v2 source and the BEBIG (60)Co source for HDR brachytherapy using the EGSnrc monte carlo transport code. J Med Phys. 2012;37(4):219–225.
- Baltas D, Lymperopoulou G, Zamboglou N. On the use of HDR 60Co source with the MammoSite radiation therapy system. Med Phys. 2008;35(12):5263–5268.
- Tamihardja J, Lutyj P, Kraft J, et al. Two-Weekly High-Dose-Rate brachytherapy boost after external beam radiotherapy for localized prostate cancer: long-term outcome and toxicity analysis. Front Oncol. 2021;11:764536.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
- Baltas D, Kolotas C, Geramani K, et al. A conformal index (COIN) to evaluate implant quality and dose specification in brachytherapy. Int J Radiat Oncol Biol Phys. 1998;40(2):515–524.
- Henry A, Pieters BR, Andre Siebert F, et al. GEC-ESTRO ACROP prostate brachytherapy guidelines. Radiother Oncol. 2022;167:244–251.
- Strohmaier S, Zwierzchowski G. Comparison of (60)Co and (192)Ir sources in HDR brachytherapy. J Contemp Brachytherapy. 2011;3(4):199–208.
- Palmer A, Hayman O, Muscat S. Treatment planning study of the 3D dosimetric differences between Co-60 and Ir-192 sources in high dose rate (HDR) brachytherapy for cervix cancer. J Contemp Brachytherapy. 2012;1(1):52–59.
- Yadav S, Singh OP, Choudhary S, et al. Estimation and comparison of integral dose to target and organs at risk in three-dimensional computed tomography image-based treatment planning of carcinoma uterine cervix with two high-dose-rate brachytherapy sources: 60Co and 192Ir. J Cancer Res Ther. 2021;17(1):191–197.
- Mosalaei A, Mohammadianpanah M, Omidvari S, et al. High-dose rate brachytherapy in the treatment of carcinoma of uterine cervix: twenty-year experience with cobalt after-loading system. Int J Gynecol Cancer. 2006;16(3):1101–1105.
- Dayyani M, Hoseinian-Azghadi E, Miri-Hakimabad H, et al. Radiobiological comparison between cobalt-60 and iridium-192 high-dose-rate brachytherapy sources: part I-cervical cancer. Med Phys. 2021;48(10):6213–6225.
- Tantivatana T, Rongsriyam K. Treatment outcomes of high-dose-rate intracavitary brachytherapy for cervical cancer: a comparison of Ir-192 versus Co-60 sources. J Gynecol Oncol. 2018;29(5):e86.
- Hoskin PJ, Rojas AM, Ostler PJ, et al. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: mature 12-year results. Radiother Oncol. 2021;154:214–219.
- Demanes DJ, Rodriguez RR, Schour L, et al. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys. 2005;61(5):1306–1316.
- Martell K, Mendez LC, Chung HT, et al. Results of 15 Gy HDR-BT boost plus EBRT in intermediate-risk prostate cancer: analysis of over 500 patients. Radiother Oncol. 2019;141:149–155.
- Strouthos I, Chatzikonstantinou G, Zamboglou N, et al. Combined high dose rate brachytherapy and external beam radiotherapy for clinically localised prostate cancer. Radiother Oncol. 2018;128(2):301–307.
- Vigneault E, Mbodji K, Magnan S, et al. High-dose-rate brachytherapy boost for prostate cancer treatment: different combinations of hypofractionated regimens and clinical outcomes. Radiother Oncol. 2017;124(1):49–55.
