Introduction
Oncogenic alterations in rearranged during transfection (RET) gene have been described in a variety of human cancers including thyroid, lung, pancreas and salivary gland malignancies [Citation1,Citation2]. The most common alterations include rearrangement, in which the RET fusion partners act as dimerization units, leading to ligand-independent homodimerization or gain-of-function mutations, directly affecting the extracellular or kinase domain. Both alterations induce ligand-independent constitutive activation of the RET receptor, followed by aberrant stimulation of downstream signaling pathways promoting growth and inhibition of apoptosis [Citation3].
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor derived from parafollicular C cells and comprise 1%–2% of thyroid tumors. Germline RET mutations are detected in multiple endocrine neoplasia 2 A and 2B subtypes, as well as in familial medullary thyroid carcinoma (FMTC) [Citation4]. Sporadic MTC accounts for approximately 80% of MTC, often harboring a poorer prognosis [Citation5,Citation6]. While FMTC comprises 20% of MTC, the majority of familial (95%) and approximately half of sporadic MTC present with somatic RET alterations [Citation7].
Until recently the therapeutic approach of advanced MTC involved multi-targeted tyrosine kinase inhibitors vandetanib [Citation8] and cabozantanib [Citation9], demonstrating notable improvement in PFS and biochemical response, although associated with significant toxicity and limited tolerability. Various side-effects are common across these multitargeted kinase inhibitors, owing to their wide-spread activity, whereas selective inhibitors often display less treatment-related toxicity.
A major advancement in the treatment of RET-driven tumors was the introduction of oral tyrosine kinase selective RET inhibitors. Pralsetinib and selpercatinib were FDA approved for the treatment of lung and thyroid cancers with RET gene mutations or fusions. The approval was based on the LIBRETTO-001 and ARROW trials, both demonstrating an overall response rate as high as 70% among treatment-naive patients with RET-mutant and fusion NSCLC and MTC [Citation10–12].
Of the 531 patients treated with selpercatinib in the LIBRETTO-001 trial, among the thyroid patients (n = 162), 28% had a grade 3 treatment-related adverse event. The adverse-event profile in patients with RET-altered thyroid cancer was similar to the overall safety profile in the 531 patients treated with selpercatinib. Across the safety cohort, the most common grade 3 treatment-related adverse events included hypertension (11%), increased alanine aminotransferase level (7%), increased aspartate aminotransferase level (5%), electrocardiogram QT-prolongation (3%), and diarrhea (2%). Two-percent had grade 4 treatment-related events, owing to increased transaminase levels. Two-percent discontinued selpercatinib secondary to drug-related toxicity. Nineteen patients had grade 5 adverse events, but all were deemed by investigator unrelated to treatment [Citation10].
Among 142 patients with RET-altered thyroid cancer treated with pralsetinib in the ARROW trial, 47% had grade 3 treatment-related adverse events, the most common (≥5%) grade 3 events included hypertension (17%), neutropenia (13%), lymphopenia (11%), anemia (10%), asthenia (4%), increased blood creatine phosphokinase (4%), pneumonitis (3%), diarrhea (2%), and electrocardiogram QT-prolongation (1%) [Citation11]. Eight (6%) had grade 4 adverse events including neutropenia (1%), thrombocytopenia (1%), lymphopenia (1%), increased blood creatinine phosphokinase (1%), and acute respiratory distress syndrome (1%). One patient (1%) died secondary to treatment-related toxicity, diagnosed with interstitial pneumonitis. In the ARROW trial, four percent discontinued the treatment secondary to the treatment side effect profile. Importantly, no CNS-related side effects have been reported with either drug.
We report on two patients treated with pralsetinib given as compassionate use for advanced MTC who developed an acute confusional state likely related to the drug. To our knowledge, this is the first report of such an association.
Case presentations
Case 1
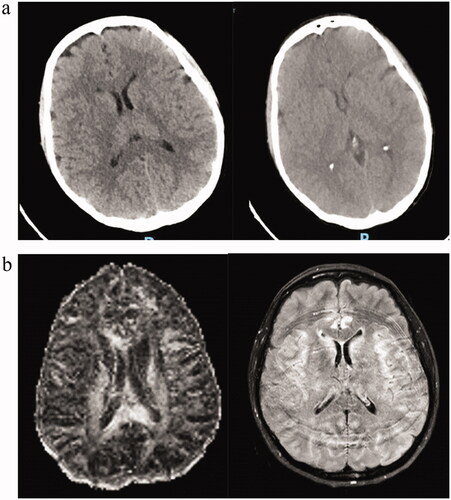
A 44-year-old female patient with no concurrent diseases, was diagnosed in 2005 with RET fusion FMTC involving bone, liver and lung metastases. She initially underwent total thyroidectomy followed by close follow-up, with slow progression of the disease over several years, while treated with zoledronic acid, calcium and vitamin D supplements to prevent skeletal related adverse events. Due to exacerbation of her symptoms of diarrhea and weight loss and concomitant increasing calcitonin levels, she received vandetanib for a period of only 9 months that was discontinued following infection with tuberculosis during a trip to Asia, and treated with eradication of the bacterium. The cancer stabilized until April 2018, when an 18F-FDOPA PET/CT revealed progression of liver metastasis, coinciding with increased diarrhea, and markedly elevated calcitonin levels. She underwent trans-arterial chemoembolization to liver metastases, with disease stabilization. In October 2021 the patient presented with symptomatic progression, characterized by severe secretory diarrhea, anorexia, and concomitant increasing calcitonin and CEA levels and radiological progression. At the end of October 2021, pralsetinib treatment was initiated as compassionate therapy, at the recommended dose of 400 mg once daily. One week following treatment initiation, the patient presented to the emergency room with an acute confusional state, severe fatigue, psychomotor disturbance, and anterograde amnesia. Neurological examination demonstrated confusion, lack of orientation to time and place, difficulty in word recall, slow tangential speech, inappropriate use of words, aphasia and altered visual perception. A MoCA test for detecting mild cognitive impairment upon admission was 24/30 (normal cognitive function is 26/30). Blood count and electrolytes levels were unremarkable. Blood chemistry revealed a marked drop in albumin (2.6 gr/dL from a baseline of 3.5 gr/dl), grade 1 elevation of alkaline phosphatase (149 IU/L from baseline of 122 IU/L), aspartate aminotransferase (97 IU/L from baseline 46 IU/L), alanine aminotransferase (35 IU/L from baseline 18 IU/L), and grade 3 asymptomatic elevation of lipase and amylase (>5.0 × ULN and asymptomatic). Head CT was unremarkable but limited by various artifacts secondary to involuntary movements (). MRI under sedation did not show brain metastases or leptomeningeal spread, but did reveal a change in signal in the medial aspect of the temporal lobe bilaterally, raising the possibility of limbic encephalitis (). Lumbar puncture was normal and demonstrated clear fluid, elevated opening pressure of 80 mm H2O, absence of polymorphonucleocytes, glucose level of 53 mg/dL, and a protein level of 29.36 mg/dL; no tumor cells were identified. A PCR viral panel and cryptococcal antigen test were negative. A neuronal autoimmune antibody screening in the blood (Neuronal NMDR, CASPR2, AMPAR1, LG1, AMPAR2, GABAB Ab) and in the CSF (Neuronal NMDR, CASPR2, AMPAR1, LG1, AMPAR2, GABAB, amphiphysin, CV2, PNMA2 Ri Ab) were also negative. Upon admission, pralsetinib treatment was immediately discontinued. Three days following suspension of treatment, progressive improvement of cognitive function was noted, with a MOCA score of 26/30. Three days following discontinuation of treatment she returned almost to her baseline state but still suffered from difficulty in concentration, with mild impairment in memory and recall. As of February 2022, she has returned to her baseline neurological state.
Case 2
A 75 year-old female with a history of hypertension, diabetes mellitus and fibromyalgia, was diagnosed in 2014 with MTC and underwent total thyroidectomy and lymph node dissection (T2N0, 2.4 cm). Following thyroidectomy calcitonin and CEA were persistently elevated (824 pmol/L, 35 ng/L, respectively), and since calcitonin measures have progressively increased.
In December 2014 a left upper lobe nodule, measuring 1 cm, was noted and biopsied, with pathology consistent with pulmonary carcinoid with a Ki67 proliferation index of less than 1%. Additionally, in May 2015 she underwent right upper lobe lobectomy for stage 1 non-small cell lung cancer adenocarcinoma (T1, 1 cm) and non-mucinous adenocarcinoma in situ (1.7 cm)
In July 2021 worsening clinical symptoms, radiological (bone and liver metastases) and laboratory progression were noted, with elevated CEA and calcitonin, and in August 2021, treatment with pralsetinib (400 mg once a day) was commenced. Within six weeks, calcitonin decreased by three-fold from 1,655 pmol/L to 500 pmol/L. However, within six weeks following initiation of therapy the patient presented twice to the ER with general deterioration, confusion, severe fatigue, nausea, vomiting, anorexia, loss of six kilograms within a week, diarrhea, loss of sphincter control and a rash upon her chest. There were no notable signs of infection, fever or evidence of a vascular event.
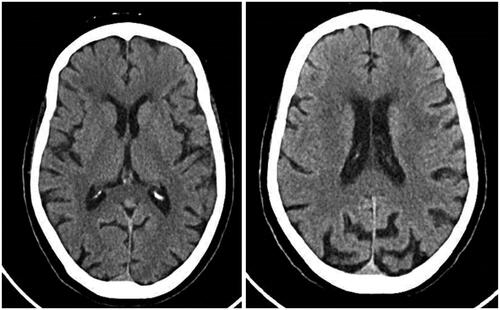
A head CT was unremarkable (), complete blood count was normal and blood chemistry unremarkable, apart from mild grade 1 hyponatremia (132 mmol/L) and a marked drop in albumin (2.78 g/dL from a baseline of 4.02 g/dL). Pralsetinib was immediately discontinued and gradually, over a period of a few weeks, her condition improved. As of January 2022 she still suffers from severe fatigue, but the confusion remitted within days of therapy discontinuation. Upon cessation of treatment with pralsetinib, calcitonin (2,367 pmol/L) and CEA levels increased (54.6 ng/mL).
Discussion
We report on two patients with metastatic RET-positive MTC who presented with acute confusional state shortly following commencing treatment with pralsetinib. In both cases no other clear explanation for the confusion was identified and improvement was noted shortly following treatment cessation, without any further intervention.
With the recent integration of pralsetinib in the treatment paradigm of RET mutant malignancies, the neurological adverse effects have yet to be delineated in the literature. Overall, pralsetinib is generally well-tolerated with a manageable safety profile.
According to the WHO definitions, the association between pralsetinib and acute confusional state can be defined at least as highly possible and in Case 1 can even be considered probable [Citation13]. The clinical symptoms and time relationship to the drug administration, the acute presentation and lack of any other identifiable causes, as well as the reversibility of the symptoms with drug cessation, make the diagnosis of pralsetinib-induced confusional state highly probable. The two patients initiated the therapy with normal neurological function and have returned to their baseline state prior to treatment; the acute neurological compromise resolved after drug withdrawal.
The MRI of patient case 1 suggested a possible etiology of limbic encephalitis, which characterizes several autoimmune conditions distinguished by inflammation of the limbic system. Cardinal features of limbic encephalitis are confusion, psychiatric symptoms, and amnesia. Limbic encephalitis is commonly associated with underlying cancer and in some cases non-paraneoplastic with a favorable response to immunosuppressive treatment, while some subtypes also present with normal CSF [Citation14]. Case 2 presented with acute confusion which could not be completely attributed to the mild hyponatremia without resolution following correction of the hyponatremia.
Both patients’ acute confusional state elicited a comprehensive neurological assessment and brain imaging ruling out metastasis, leptomeningeal disease, infectious and vascular etiologies. The patients developed an acute confusional state but with different presentations and some limitations in the work-up. Case 1 was a young female, who was well examined and other causes of confusion were excluded by a comprehensive assessment, while Case 2 was an older female, who did not complete a brain MRI, to exclude leptomeningeal disease. Lumbar puncture was not performed ruling-out encephalitis, as there were no clinical or laboratory measures prompting suspicion of viral or bacterial infection. Although the confusional state of Case 2 may have been caused by diarrhea, vomiting and dehydration, the blood work did not reflect an electrolyte imbalance supporting such an etiology. Additionally, Case 2 also recovered much more slowly than Case 1. Acute confusion among patients treated with pralsetinib prompts a thorough neurological evaluation including complete blood count, comprehensive metabolic panel, blood gases, head CT, MRI, and lumbar puncture excluding other causes.
The two patients did not receive concomitant medications which could have enhanced the toxicity of pralsetinib or could have induced altered neurological function. Both patients’ anamnesis ruled out ingestion of alcohol or drugs. The coadministration of pralsetinib with strong CYP3A inhibitors may increases pralsetinib exposure, heightening the severity and incidence of adverse effects. Mild hepatic impairment (total bilirubin ≤1.0 × ULN and AST > ULN, or total bilirubin >1.0 to 1.5 × ULN and any AST) has no effect on the pharmacokinetics of pralsetinib, while patients with moderate and severe hepatic impairment have not been studied with pralsetinib. The patients had normal to mildly elevated liver function; additionally, case 1 had an unexplained and transient increase in the pancreatic enzymes. Interestingly, both patients treated with pralsetinib had liver metastases, and had a marked acute decrease in albumin with the onset of the confusion, which returned to normal range after the drug was discontinued. Hypoalbuminemia may be associated with pralsetinib-induced confusion. Hypoalbuminemia is often a measure of critical illness, while the drop in the biomarkers in Case 2 do not support progressive disease, the elevated transaminases with the concomitant hypoalbuminemia are suggestive of a possible confusion stemming from hepatic induced injury. Additionally, ammonia was normal in the blood work of both patients.
In the ARROW study approximately 40% of patients treated had known brain metastasis [Citation15], with confirmed responses in the intracranial lesions. Some patients even achieved CNS complete response, supporting the drugs penetration of the blood brain barrier, suggesting a possible central mechanism of the drug elicited confusion.
Recognition of pralsetinib-induced acute confusional state is extremely important both for rapid discontinuation of the drug and avoidance of possible irreversible compromise. Incremental dose escalation in accordance with patient tolerability, or initiation of the drug at lower dosages, with slow dosage optimization, may be considered and may provide an optimal approach to monitoring and preventing serious adverse effects. The phase I ARROW trial involving dose escalation of pralsetinib, demonstrated no drug limiting toxicity with lower doses.
It may be too soon to suggest rechallenge with careful dose escalation after complete resolution of symptoms, before more is known about this toxicity. These cases encourage a high degree of caution and increased awareness of possible neurological deterioration when administering pralsetinib among caretakers, and a thorough neurological assessment should be undertaken. With increased administration of pralsetinib, additional similar reports may shed light on the incidence, clinical significance, and the mechanism of this rare, but significant, drug adverse effect.
Acknowledgements
Roche for providing compassionate supply of pralsetinib.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Amit M, Na'ara S, Fridman E, et al. RET, a targetable driver of pancreatic adenocarcinoma. Int J Cancer. 2019;144(12):3014–3022.
- Takahashi M, Kawai K, Asai N. Roles of the RET proto-oncogene in cancer and development. JMA J. 2020;3(3):175–181.
- Wagner S, Zhu S, Nicolescu A, et al. Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2. Clinics. 2012;67(S1):77–84.
- Wells SA, Pacini F, Robinson BG, et al. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98(8):3149–3164.
- Pelizzo MR, Boschin IM, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007;33(4):493–497.
- Leboulleux S, Baudin E, Travagli JP, et al. Medullary thyroid carcinoma. Clin Endocrinol (Oxf). 2004;61(3):299–310.
- Taccaliti A, Silvetti F, Palmonella G, et al. Genetic alterations in medullary thyroid cancer: diagnostic and prognostic markers. Curr Genomics. 2011;12(8):618–625.
- Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141.
- Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646.
- Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–835.
- Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491–501.
- Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–969.
- The use of the WHO-UMC system for standardised case causality assessment. Accessed February 5, 2022. https://www.who.int/publications/m/item/WHO-causality-assessment.
- Blinder T, Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-a systematic analysis. Front Neurol. 2019;10:804.
- Gainor JF, Lee DH, Curigliano G, et al. Clinical Activity and Tolerability of BLU-667, a Highly Potent and Selective RET Inhibitor, in Patients with Advanced RET-Fusion + Non-small Cell Lung Cancer. 2019 ASCO Annual Meeting.