?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Exercise during oncological treatment is beneficial to patient health and can counteract the side effects of treatment. Knowledge of the societal costs associated with an exercise intervention, however, is limited. The aims of the present study were to evaluate the long-term resource utilisation and societal costs of an exercise intervention conducted during (neo)adjuvant oncological treatment in a randomised control trial (RCT) versus usual care (UC), and to compare high-intensity (HI) versus low-to-moderate intensity (LMI) exercise in the RCT.
Methods
We used data from the Physical Training and Cancer (Phys-Can) project. In the RCT, 577 participants were randomised to HI or to LMI of combined endurance and resistance training for 6 months, during oncological treatment. The project also included 89 participants with UC in a longitudinal observational study. We measured at baseline and after 18 months. Resource utilisation and costs of the exercise intervention, health care, and productivity loss were compared using analyses of covariance (RCT vs. UC) and t test (HI vs. LMI).
Results
Complete data were available for 619 participants (RCT HI: n = 269, LMI: n = 265, and UC: n = 85). We found no difference in total societal costs between the exercise intervention groups in the RCT and UC. However, participants in the RCT had lower rates of disability pension days (p < .001), corresponding costs (p = .001), and pharmacy costs (p = .018) than the UC group. Nor did we find differences in resource utilisation or costs between HI and LMI exercise int the RCT.
Conclusion
Our study showed no difference in total societal costs between the comprehensive exercise intervention and UC or between the exercise intensities. This suggests that exercise, with its well-documented health benefits during oncological treatment, produces neither additional costs nor savings.
Introduction
Despite advances leading to increased survival rates, oncological treatment continues to be associated with side effects leading to substantial use of health care resources and corresponding costs [Citation1,Citation2]. The side effects of cancer and its treatment also negatively affect survivors’ work productivity [Citation1,Citation3–6], leading to a greater societal costs than the medical expenditures alone [Citation1,Citation2,Citation7,Citation8]. Investments in cancer rehabilitation are therefore important to decrease the societal costs of cancer [Citation9].
There is strong evidence that exercise during oncological treatment counteracts side effects and is more effective than usual care in preventing declines in health-related quality of life (HRQoL) [Citation10–15], reducing hospitalisations [Citation16–18], and improving return to work [Citation19–21] and may therefore reduce costs. Previous exercise trials also indicates that high-intensity exercise may be more beneficial than low-intensity in improving physical functioning [Citation10] and reducing physical fatigue [Citation21]. In a recent RCT in the Phys-Can project comparing supervised programmes of high-intensity (HI) versus low-to-moderate intensity (LMI) exercise, we found small but significant between-group differences in muscle strength and cardiorespiratory fitness at post-intervention, and significant but not clinically relevant differences in physical fatigue (main outcome) in favour of HI exercise. We found no post-intervention differences between HI and LMI exercise in HRQoL, anxiety, depression, functioning in daily life, or sleep [Citation22]. Thus, we could not conclude that the exercise intensity is of major importance for the benefits of exercise during oncology treatment.
Supervised exercise programmes are more expensive than unsupervised, but the supervised are more effective in improving HRQoL, anxiety, depressive symptoms, and physical functioning [Citation23]. Because resources are scarce, decision-makers need health economic evaluations to justify extra costs when choosing interventions based on their effectiveness [Citation24]; however, health economic evaluations of exercise programmes during oncological treatment are limited. Studies also rarely include reliable register data on sick leave. A systematic review focussed mainly on the short-term cost-effectiveness of multidimensional cancer rehabilitation programmes, including exercise, concluded that interventions that improved overall health might be cost-effective [Citation25]. Another systematic review including exercise interventions with longer follow-up considered three of the interventions in the seven studies included cost-effective [Citation26]. High-intensity exercise programmes also appear more cost-effective than usual care [Citation27,Citation28] and low-to-moderate intensity programmes [Citation29]. Thus, evidence for the cost-effectiveness of exercise remains inconclusive [Citation25,Citation26,Citation28], and cost calculations and outcome measures are heterogenous across studies. To attain more robust results, further research is needed to evaluate the longer term costs of exercise intervention programmes and the differences in costs between different exercise intensities.
In the present study, we investigated resource utilisation and costs at longer term (i.e. 18 months), using data from the Physical Training and Cancer (Phys-Can) project. The main component of the Phys-Can project is an RCT (Clinical Trials NCT02473003, www.clinicaltrials.gov) comparing the effects of HI versus LMI exercise with or without additional behaviour change support (BCS) in individuals undergoing (neo)adjuvant oncological treatment. We then compared the results of the RCT with a preceded longitudinal observational study of participants from the same population treated with usual care (UC) [Citation30].
The aims of the present study were to evaluate the long-term resource utilisation and societal costs of an exercise intervention during (neo)adjuvant oncological treatment in a randomised control trial (RCT) versus UC, and to compare HI with LMI exercise in the RCT.
Table 1. Baseline sociodemographic and clinical characteristics in patients undergoing (neo)adjuvant oncological treatment.
Methods
Research design and study sample
Participants with breast, colorectal, or prostate cancer, aged ≥18 years and scheduled for neoadjuvant and/or adjuvant oncology treatment, were recruited at three university hospitals in Sweden from September 2014 to March 2015 (UC) and March 2015 to May 2018 (RCT). Patients unable to perform basic activities of daily living or with other conditions that might contraindicate physical exercise (e.g. heart failure, chronic obstructive pulmonary disease, orthopaedic conditions, or neurological disorders) were excluded. After completion of baseline measurements, participants in the RCT were stratified by cancer diagnosis and hospital and randomly assigned to one of four exercise interventions: LMI, LMI with BCS, HI, and HI with BCS.
We considered it unethical to randomise participants to a UC control group in the RCT since strong evidence indicates the benefits of exercise during oncological treatment. Therefore, we designed a longitudinal observational study with participants receiving UC, before the RCT started. The participants in UC did not participate in any exercise programme and were evaluated with the same outcome measures as those in the RCT. Full details of the study design are published elsewhere [Citation30]. This study was approved by the Swedish Ethical Review Authority in Uppsala, Sweden (Dnr 2014/249) and conducted in accordance with the Helsinki Declaration. Informed consent was obtained from all participants.
The exercise intervention
The exercise intervention consisted of home-based endurance training and supervised resistance training for 6 months during oncological treatment according to standardised protocols; details presented elsewhere [Citation22,Citation30]. Coaches (physiotherapists and personal trainers) were educated to provide the intervention. For the endurance training, the HI group performed two weekly sessions of interval training (2-min intervals and 2-min active rest periods) for a total of up to 75 min per week, typically running or cycling. The LMI group performed at least 150 min per week of e.g. walking, in bouts of at least 10 min. The resistance training, supervised in a group and performed twice a week at a public gym, included both machine (seated leg press, chest press, leg extension, seated row, seated leg curl, and seated overhead press using dumbbells) and core strengthening (sit-ups, the plank, bird-dog, and pelvic floor exercises) exercises. The HI group performed 3 sets × 6 repetitions maximum (RM) with 2 min set rest in the first weekly session and 3 sets × 10 RM with 1 min set rest in the second weekly session. The LMI group performed 3 sets × 12 repetitions at 50% of 6 RM with 2 min set rest in the first weekly session and 3 sets × 20 repetitions at 50% of 10 RM with 2 min set rest in the second weekly session. Half of the participants in the HI and LMI groups were provided additional BCS, extra support strategies to facilitate adherence to the endurance training and maintain physical activity after completion of the exercise intervention. The exercise guided by the coaches was monitored by research staff to demonstrate fidelity to the protocol. The exercise performed by the participants was monitored by the coaches to register adherence to the protocol.
Measures
Cost analyses were performed according to Drummond et al. [Citation24] with the use of a societal perspective. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist was applied to guide our reports of the methods and results [Citation31].
Background characteristics
Sociodemographic data and comorbidities were self-reported at baseline. Medical background data were collected from the medical records and the Swedish National Quality Register.
Resource utilisation and its corresponding cost
Data were obtained for 6 months before baseline measurement (prior to the start of the oncological treatment and the exercise intervention) and up to 18 months after. Societal costs comprised the costs of the exercise intervention, the participants’ health care, and any loss of productivity. The exercise intervention cost and reimbursement of out-of-pocket money are detailed in the Supplementary Material. Costs included labour costs for the coaches (including their education and exercise supervision); time worked × gross hourly wages + overhead, fitness centre membership fees, maximal oxygen uptake ( O2 max) testing (estimated from invoices), and costs of heart rate monitors (estimated from market prices). Travel expenses were considered out-of-pocket money for the participants, and compensated by mileage according to the Swedish Tax Agency 2019 [Citation32]. In Sweden, health care is financed through taxes (and minor user co-payments) and is part of the welfare system. Health care in this study included outpatient visits (except for primary care), hospitalisation, and prescribed medication. Health care utilisation data were retrieved from the Swedish National Board of Health and Welfare [Citation33]. Each visit to health care was coded according to the Swedish NordDRG pricelists [Citation34]. Cost of prescribed medications was estimated using market prices [Citation35]. Costs related to productivity loss we calculated by days absent from paid work. Sick leave and disability pension days were obtained from the Swedish Social Insurance Agency [Citation36]. In Sweden, the first 14 days of sick leave are paid by the employer, which means that we do not have data on sick leave periods shorter than 15 calendar days, but we did add in the first 14 days for sick leave episodes longer than that. Productivity losses were valued according to the human capital approach, using the mean productivity cost of full-time workers including all taxes and social fees from 2019 (€4550) [Citation37] and recalculated as full-time equivalent days. We did not discount costs because the total study time was under 2 years. Cost was converted from SEK to Euros using an exchange rate of €1 = SEK 9.963 as of 28 October 2021 [Citation38].
Statistical analyses
The analyses were performed in IBM SPSS statistics 25 according to the intention-to-treat-principle; p ≤ .05 was considered statistically significant. Descriptive background characteristics are presented as mean, standard deviation, and frequencies, and groups are compared using t tests for continuous data and Chi-squared tests for categorical data. Analyses of covariance (ANCOVA) were used to compare differences in resource utilisation and total and disaggregated costs between the RCT and UC. Since the UC group was not randomised and included participants before the RCT started, we adjusted for possible confounders. Baseline measurements 6 months before each outcome and age were included as covariates while sex and chemotherapy [yes/no]) were included as fixed factors in the models. Independent t tests were used to compare differences in resource utilisation and total and disaggregated costs within the RCT (HI vs. LMI).
Results
Participants
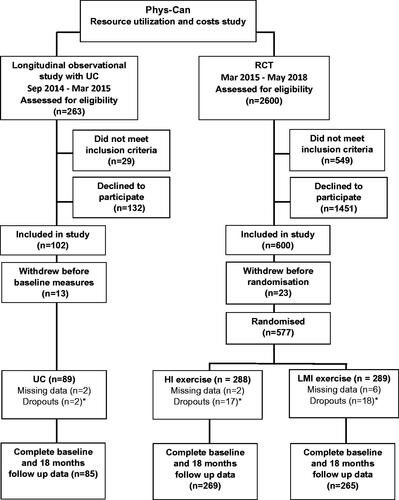
Complete data on resource utilisation and costs were available for 619 participants in the Phys-Can project (RCT HI: n = 269, LMI: n = 265, and UC: n = 85); data from 10 participants were randomly missing (). There were no statistically significant differences in the background characteristics between the groups, except in women with breast cancer, a smaller proportion of whom in UC received chemotherapy those in the RCT (p < .001). The mean age of all participants were 59 years, and the majority (80%) were women with breast cancer. More than half had a university education, over 60% were employed, and about a third were retired (). Participants attended on average 25 (48%) of the supervised exercise sessions in the RCT. At baseline (measured over a 6-month period before inclusion in the studies), the RCT had significantly lower costs of outpatient visit (p < .001) and units of outpatient visits (p = .041) than UC. The costs of health care and productivity loss and the total societal costs did not differ significantly between the HI and LMI exercise interventions.
Figure 1. CONSORT flow chart of participants through the Phys-Can costs and resource utilisation study, including the Phys-Can longitudinal observational study of UC and the Phys-Can RCT. UC: usual care; HI: high-intensity exercise; LMI: low-to-moderate intensity exercise. *Dropouts who withdrew consent during the study time up to 18 months.
Resource utilisation
The RCT versus UC
At the 18-month follow-up, participants in the RCT had a lower rate of disability pension days than those who had had UC (diff: −9.5; confidence interval [CI] 95% −15 to −4.2). No other significant differences were found between the groups, but participants in the RCT tended to have fewer outpatient visits and more sick leave days and corresponding costs than those under UC ().
Table 2. Resource use per participant in the RCT versus UC and HI versus LMI at the 18-month follow.
HI versus LMI exercise within the RCT
There were no significant differences in health care resource utilisation or productivity loss between the exercise intensities at the 18-month follow-up ().
Costs
The largest costs in both the RCT and UC were driven by health care utilisation and productivity losses. The average cost for providing the exercise intervention (52 sessions) was estimated at €2,622 per participant (for both HI and LMI). The largest cost of the intervention was wages for exercise supervision by the coaches. A detailed description of the costs of the exercise intervention is presented in the Supplementary Material.
The RCT versus UC
The total societal costs per participant (€35,253 in the RCT and €32,338 under UC) did not differ significantly at the 18-month follow-up. The pharmaceutical costs were significantly lower in the RCT than under UC (diff: −254€; 95% CI: −466 to −43); however, UC had one outlier with pharmacy costs of €16,100. The disability pension costs were significantly lower in the RCT than under UC (diff: −€1425, 95% CI: −2230 to −621). No other significant differences were found between the RCT and UC ().
Table 3. Costs and cost differences of RCT versus UC and HI versus LMI at the18-month follow-up (€).
HI versus LMI exercise within the RCT
Total cost of HI was €35,519 per participant versus €33,387 for LMI at the 18-month follow-up. No significant differences were found between the exercise intensities in any cost category ().
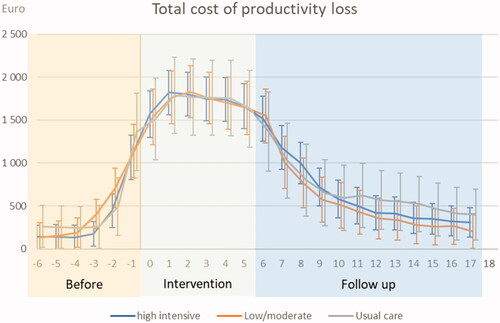
The total monthly costs of productivity loss over the study period were similar between both RCT groups and UC. A large increase in costs during the intervention decreased during the follow-up period ().
Discussion
We evaluated the longer term (18 months) resource utilisation and societal costs of a 6-month exercise intervention during oncological treatment. Participants in the RCT (exercise intervention) had lower rates of disability pension days, and pharmacy costs than the UC group No significant differences in total societal costs were found between the RCT and UC or between the intensity groups (HI vs. LMI) in any category of resource utilisation or cost.
The finding that total societal costs did not differ between the RCT and UC was in line with previous research by van Waart et al. who evaluated a home-based low-intensity walking program with a combined supervised endurance and resistance exercise program on moderate to high-intensity in patient with breast cancer receiving chemotherapy compared with usual care [Citation27]. However, in our study the follow-up was 12 months post-intervention compared with 6 months in van Waart et al. study. This indicates that our comprehensive exercise programme of endurance and supervised resistance training along with additional BCS did not add any long-term costs from a societal perspective. This finding is important since exercise during oncological treatment has proven to be beneficial for health and HRQoL in patients with cancer [Citation23]. However, a smaller trial by May et al. of an 18 weeks’ supervised exercise intervention with a shorter follow-up showed that the total societal costs were lower for patients with colon cancer and higher for those with breast cancer compared with usual care [Citation18]. Thus, comparisons with other studies may be difficult due to different cancer populations, health systems, payment structures, intervention contents and characteristics, and times to follow-up. We suggest further research focussed on the cost-effectiveness of different intervention characteristics in different cancer populations.
There was no difference in total societal costs between the intensity groups in the RCT or in their long-term resource utilisation, healthcare costs, or loss of productivity. One possible reason for this might be that only small between-group differences in health-related outcomes were found directly after the exercise interventions [Citation22]. This novel information expands the knowledge from previous studies [Citation27,Citation29] since our study have directly compared two exercise intensities during oncological treatment with longer follow-up. In contrast to our findings, however, Kampshoff et al. [Citation29]. found lower health care costs for high-intensity than for low-intensity exercise This may be because our intervention was delivered during treatment, which has been proven more beneficial than a later start [Citation39], while theirs started after participants had already completed chemotherapy.
Although health care costs did not differ between the RCT and UC in our study, the RCT had lower pharmaceutical costs than UC, in contrast to the findings by van Waart et al. [Citation27] of no between-group differences. Our UC group, however, had one participant with outlying costs of €16,100. The removal of that participant resulted, as in van Waart et al., in no difference between the RTC and UC groups. Moreover, we found no between-group difference in hospitalisation rates for the participants, similar to the findings by May et al. findings in patient with breast cancer, although they found lower rates in patients with colon cancer in their exercise intervention group compared with usual care [Citation18]. Mijwel et al. examined a 16-week exercise intervention in patients with breast cancer receiving adjuvant chemotherapy. They found lower hospitalisations rates in their high-intensity aerobic interval training combined with resistance training (RT-HIIT) group than in the usual care group, but no differences between the usual care group and their group given moderate-intensity aerobic training combined with high-intensity interval training (AT-HIIT) [Citation16]. Nevertheless, van Waart et al. found higher costs related to hospitalisation and outpatient visits, but lower costs for primary care, in their low-intensity exercise group than in their usual care group [Citation27]. The different costs and usages of health care resources in these studies may be explained by their different health systems, payment structures, intervention characteristics, cancer populations, and follow-up times. More research is needed to confirm our findings.
Our study also adds knowledge about long-term productivity loss after commencement of oncological treatment. We found no difference in costs related to productivity loss or rates of sick leave between the RCT and UC, which accords with the findings of van Waart et al. [Citation27]. The loss-of-productivity costs were higher during our intervention due to participants’ ongoing oncological treatment. We had expected the RCT to have lower productivity losses than UC, but because our supervised group resistance training was scheduled during the daytime, some participants were required to take sick leave to participate. Contrary to our findings, Mijwel et al. reported a lower proportion of sick leave in their AT-HIIT group, but no difference between their RT-HIIT and usual care [Citation40], although their participants trained individually and hence had more flexible schedules than our supervised groups. The study by May et al. also found higher costs in their exercise intervention for sick leave in patients with breast cancer, but lower costs for patients with colon cancer, than for those in the usual care group [Citation18]. The data on productivity loss, however, is more reliable in our study since we included register data, while other studies measured sick leave from self-reported diaries over a shorter period of time after the exercise intervention. Our study also had some other important differences from previous studies, including lower disability pension days (and hence lower disability pension costs) in the RCT than in the UC group. These findings cannot be compared with other studies since they registered absences from work as sick leave days only. We did not find any between-group differences in comorbidities that could explain these findings. Hence, more research is needed to confirm these findings.
The costs of the intervention in the present study were higher than in comparable supervised exercise intervention studies [Citation18,Citation27,Citation29], as our intervention was more comprehensive, lasted longer, and consisted of both exercise and additional BCS, resulting in higher labour costs. We will therefore further examine the long-term effectiveness of our intervention in relation to its costs in future studies.
Methodological considerations
Strengths of the present study included our relatively large sample from a multicentre RCT directly comparing HI and LMI exercise, long-term follow-up (i.e. 18 months), use of reliable cost and resource utilisation measures from national registers with few missing data, and the rigour of the exercise programme, which followed a strictly standardised protocol.
However, some limitations must be considered when interpreting our results. The main limitation was our non-randomised smaller sample of participants under UC before the RCT, which could introduce bias to the results. However, the inclusion of UC participants stopped immediately before the RCT started so the difference in time between the groups was negligible, and the oncological treatment regimens were similar in both groups. The background characteristics were similar between the groups, except that a smaller proportion of participants with breast cancer received chemotherapy in the UC group. Because of this limitation, we adjusted for possible confounders in our analyses. Other potential limitations were that we did not include the possible costs of participants’ informal care, unpaid productivity (e.g. volunteer work), or reduced productivity at work, although it is likely that the positive effect of the exercise intervention facilitated productivity while working. Other limitations in the calculation of costs were the lack of data on visits to the primary care as well as lack of non-prescribed drugs, and short-term sick leave. This study included a study population relatively healthier than the general cancer population, most of whom were higher educated women with breast cancer. Thus, the generalisability to other cancer population might be limited. Our results also might not be applicable to other countries with different health care systems and/or payment structures.
Conclusion
Our study showed no long-term difference in total societal costs between the comprehensive exercise intervention and UC. Although this result indicates that exercise, with its well-documented health benefits during oncological treatment, can be implemented without additional costs to society, it also suggests that cost reductions cannot be expected. Resource utilisation and societal costs did not differ between the exercise intensities, indicating that both HI and LMI may be considered in cancer care according to individuals patients’ preferences. Further research is warranted to evaluate the long-term cost-effectiveness of exercise interventions during oncological treatment and in different cancer populations.
Supplemental Material
Download MS Word (71.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Carlotto A, Hogsett VL, Maiorini EM, et al. The economic burden of toxicities associated with cancer treatment: review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics. 2013;31(9):753–766.
- Jönsson B, Hofmarcher T, Lindgren P, et al. The cost and burden of cancer in the European union 1995–2014. Eur J Cancer. 2016;66:162–170.
- de Boer AGEM, Verbeek JHAM, Spelten ER, et al. Work ability and return-to-work in cancer patients. Br J Cancer. 2008;98(8):1342–1347.
- Lundh MH, Lampic C, Nordin K, et al. Sickness absence and disability pension following breast cancer – a population-based matched cohort study. Breast. 2014;23(6):844–851.
- Mehnert A. Employment and work-related issues in cancer survivors. Crit Rev Oncol Hematol. 2011;77(2):109–130.
- de Boer AG, Torp S, Popa A, et al. Long-term work retention after treatment for cancer: a systematic review and Meta-analysis. J Cancer Surviv. 2020;14(2):135–150.
- Ekwueme DU, Yabroff KR, Guy GP, Jr., et al. Medical costs and productivity losses of cancer survivors-United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2014;63(23):505–510.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128.
- Stout NL, Silver JK, Raj VS, et al. Toward a national initiative in cancer rehabilitation: recommendations from a subject matter expert group. Arch Phys Med Rehabil. 2016;97(11):2006–2015.
- Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2018;52(8):505–513.
- Gebruers N, Camberlin M, Theunissen F, et al. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support Care Cancer. 2019;27(1):109–122.
- Fuller JT, Hartland MC, Maloney LT, et al. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of Meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311–1311.
- Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9(9):CD005001.
- Mishra SI, Scherer RW, Snyder C. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;5(8):CD008465.
- Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):464–468.
- Mijwel S, Bolam KA, Gerrevall J, et al. Effects of exercise on chemotherapy completion and hospitalization rates: the OptiTrain breast cancer trial. Oncologist. 2020;25(1):23–32.
- Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517.
- May AM, Bosch MJ, Velthuis MJ, et al. Cost-effectiveness analysis of an 18-week exercise programme for patients with breast and Colon cancer undergoing adjuvant chemotherapy: the randomised PACT study. BMJ Open. 2017;7(3):e012187.
- de Boer A, Taskila TK, Tamminga SJ, et al. Interventions to enhance return‐to‐work for cancer patients. Cochrane Database Syst Rev. 2015;2015(9):CD007569.
- Mijwel S, Jervaeus A, Bolam KA, et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244–256.
- van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–1927.
- Demmelmaier I, Brooke HL, Henriksson A, et al. Does exercise intensity matter for fatigue during (neo-)adjuvant cancer treatment? The Phys-Can randomised clinical trial. Scand J Med Sci Sports. 2021;31(5):1144–1159.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Mewes JC, Steuten LM, Ijzerman MJ, et al. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist. 2012;17(12):1581–1593.
- Khan KA, Mazuquin B, Canaway A, et al. Systematic review of economic evaluations of exercise and physiotherapy for patients treated for breast cancer. Breast Cancer Res Treat. 2019;176(1):37–52.
- van Waart H, van Dongen JM, van Harten WH, et al. Cost-utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. Eur J Health Econ. 2018;19(6):893–904.
- Gubler-Gut BE, Pöhlmann J, Flatz A, et al. Cost-effectiveness of physical activity interventions in cancer survivors of developed countries: a systematic review. J Cancer Surviv. 2021;15(6):904–961.
- Kampshoff CS, van Dongen JM, van Mechelen W, et al. Long-term effectiveness and cost-effectiveness of high versus low-to-moderate intensity resistance and endurance exercise interventions among cancer survivors. J Cancer Surviv. 2018;12(3):417–429.
- Berntsen S, Aaronson NK, Buffart L, et al. Design of a randomized controlled trial of physical training and cancer (Phys-Can) – the impact of exercise intensity on cancer related fatigue, quality of life and disease outcome. BMC Cancer. 2017;17(1):218.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur J Health Econ. 2013;14(3):367–372.
- Swedish Tax Agency. [cited 2021. Oct 7]. Available from: https://www.skatteverket.se/privat/skatter/beloppochprocent/2021.4.5b35a6251761e6914204479.html#h-Bilersattningmilersattning
- The National Board of Health and Welfare. Registers [cited 2021 Oct 7]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/
- The National Board of Health and Welfare. Viktlistor för NordDRG: The National Board of Health and Welfare [cited 2021 Aug 20]. Available from: https://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/viktlistor/
- FASS. Farmacevtiska specialiteter i Sverige. 2020. [cited 2021 Oct 20]. Available from: https://www.fass.se
- Swedish Social Insurance Agency. [cited 2020 Dec 1]. Available from: https://www.forsakringskassan.se/statistik
- SCB statistikdatabasen. 2020. [cited 2021 Oct 7]. Available from: https://www.scb.se/hitta-statistik/sverige-i-siffror/utbildning-jobb-och-pengar/medianloner-i-sverige/
- The Riksbank (Sweden's central bank). [cited 2021 Oct 7]. Available from: https://www.riksbank.se/sv/statistik/sok-rantor-valutakurser/forklaring-till-serierna/valutakurser
- Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426.
- Bolam KA, Mijwel S, Rundqvist H, et al. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–648.